NEEP533 Course Notes (Spring 1999)
Resources from Space
Lecture #13: Legacy of the Baby Boomers: Its only a Gassy Moon!!
Resources of the Moon: Indigenous
 |
Artist's conception (Pat Rawlings) of a Lunar Base (What's wrong with this picture? No radiation protection) |
 |
Artist's (Pat Rawlings) conception of a Lunar Base (better). |
- o Having been on the surface and sampled lunar materials (ground truth), we know a great deal about the Moon's resources that could support long term settlement. Additional information has come recently from
- Galileo,
- Clementine, and
- Lunar Prospector- and
- some direct as well as indirect geochemical sensing from the Earth and Apollo lunar orbits.
 |
Figure: AS 17 152 23311 Full Moon/Full self sufficiency needed! |
| Figure: AS 17 134 20509 Living on the lunar surface |
- Factors affecting the accessibility of minerals on the Moon
- o Absence of water!!!!
- o Original rock composition
- o Igneous differentiation (less extreme than on Earth due to absence of water)
- o Regolith formation
- o Fluidized sorting
- o Other ?
- Non-metallic materials required for lunar construction
- o Regolith cover for insulation and radiation protection
- o Road aggregate (as a by-product from mining and processing of regolith)
- o Dry compacted regolith fines (Desai, 1993)
- o Sintered or cast regolith-based structural materials
- o Regolith/metal fiber composites through thermal liquefaction (Desai, 1993)
 |
Relatively undisturbed regolith surface. |
 |
Moderately disturbed regolith surface. |
Figure: AS17 134 20394 Aggregate-rich regolith near Powell Crater.- Figure: AS17 146 22429 Local layered structure to regolith at Van Serg Crater
- Metallic materials required for lunar operations and manufacturing.
 |
Lunar Soil Composition |
 |
Comparison of compositions of lunar soil and the Earth's crust |
Note: The overall availability of potential resources on a planet represents a critical baseline - if its not there, forget about it.- Locating economically extractable concentrations of such resources, however, constitutes the difference between a potential resource and a commodity.
- In the following outline, the indicated concentration (grade) of a particular resource is in relation to the concentration in its best known host.
o Native Iron-1 vol% in some mature regolith (also from meteorite debris, about 0.1% of regolith)- o Nickel and Cobalt
- o Platinum Group, Ge, Re, and other siderophile elements, e.g. Au
o TiO2-13 wt% from ilmenite (some in basaltic regolith)- o Oxygen and iron (FeO-22 wt% in ilmenite-rich Basalts) can be by-products
o Al2O3-35 wt% and SiO2-45 wt% from CaAl2Si2O8 (anorthite, the dominant mineral in lunar anorthosite)- o Silicon (Aluminum)
- o Oxygen
- o Sodium
- Note:
Silicon Production for solar cells, micro-electro-mechanical devices, and other chip applications (Seboldt, et al, 1993, in Lewis, et al, 1993)): - o Heat regolith or pyroclastic glass in the presence of F (fluorine), producing fluorosilane (SiF4)
- o Extract Si (metal) through plasma processing
- o An intermediate step involving the production of SiH4 (silane) may be required
- o Other metal fluorides can be reduced by processing with K (potassium) to recycle F if desired.
- o Cr2O3-0.5-1 wt% from (Fe,Mg)(Cr,Al,Ti)2O4 (spinel in regolith)
- o MgO- wt% from (Mg,Fe)2SiO4 (olivine)
- Note: Significant concentrations of olivine near the base of near surface basalt flows were noted and sampled at the Apollo 12 exploration site in Mare Cognitum.
- Other useful elements
- o P2O5-0.5 wt% in phosphate minerals in KREEP
- o Na2O-1.2 wt% and K2O-3.6 wt% in KREEP
- o Rare Earth Elements, Hf, and Zr concentrated in KREEP related materials
Indigenous volatiles- Oxygen from H2O derived from pyroclastics (orange and green soils) (Allen, et al, 1996)
- o Orange soil provided a yield of 5% when reacted with hydrogen at 1050oC, with over 90% of this yield in 3 hrs.
- o Yield from lunar soils, in general, is a linear and direct function of iron content
- o Chlorine and fluorine from pyroclastics (orange and green soils)
- o Zn, Mn, Cu, Pb, and other chalcophile elements if processed in large volumes.
|
|
|
|
|
|
|
Large deposits of orange soil along southwestern rim of Serenitatis |
o Sulfur from FeS (troilite) in basaltic regolith
Mineral concentrations possible in differentiated cooling units in the maria- o Titanium (ilmenite), chromium (chromite), iron and sulfur (native iron and troilite)
Resources of the Moon: Solar Wind/Cosmic Ray
Solar Wind Volatiles from the regolith
- What are the Solar Wind Volatiles?
- Hydrogen (96%)
- Helium (4%)
- Nitrogen
- Carbon
- Noble Gases (Kr, Xe, Ar)
- The solar wind is the product of fusion reactions in the sun.
The structure of the solar wind in the vicinity of the Earth and Moon is profoundly affected by the Earth's magnetic field.
| Structure of the Solar Wind in the vicinity of the Earth and Moon | |
| Relative Solar Wind exposure for the near side and far side of the Moon |
Hydrogen and helium are the principal components of the solar wind.
 |
Composition of the Solar Wind |
|
|
Applications of Lunar Volatiles |
Based on the measured concentrations in lunar samples and extrapolations
across the Moon's surface, a rough inventory of lunar volatiles in the
upper 3m of the lunar surface can be made.
| Inventory of Lunar Volatiles in first three meters of regolith (note log scale). | |
| Inventory of Lunar Volatiles relative to the production of one tonne of helium-3 |
More specific data on helium relationships:
 Measured Helium content in lunar samples by mission
Measured Helium content in lunar samples by mission Variations in solar wind volatile concentrations by as much as a factor of two have been measured as a function of depth in the regolith in three, 2 to 3 meter drill cores (Apollos 15, 16, and 17). These variations appear to be random and the result of varying maturity of superposed ejecta blankets from which the regolith has been constructed and, in the case of Apollo 17, possible zones of increased abundance of high titanium pyroclastic material.
The process of "gardening" the regolith by repeated impacts of micrometeorites clearly results in the release of some implanted volatiles, but, none the less, there is an increase in concentrations with maturity as measured by decreasing grain size.
A strong correlation exists between helium content and TiO2 (ilmenite - FeTiO3) content, and there may be an increase in concentration with increased maturity as measured by the increased reduction of iron in the regolith to iron metal over time.
| Correlation of Helium content with TiO2 and maturity (Taylor, 1973) | |
| Correlation of Helium Content with TiO2 (and mare vs. highland soils) |
The correlation of helium-3 with the product of TiO2
content and maturity, however, is very strong, at least for the samples
returned so far. (Taylor, 1993)
 Correlation of Helium content with (TiO2)x(maturity) (Taylor,
1973) "
Correlation of Helium content with (TiO2)x(maturity) (Taylor,
1973) "
Remote sensing from Earth and from the Clementine orbits can be used to
give an approximate distribution of titanium in lunar surface materials
and thus a rough indication of helium distribution, although the maturity
of the soils would need to be over-laid on this distribution to give a
better approximation.
| Distribution of lunar TiO2 as observed by the Clementine mission | |
| Distribution of TiO2 as observed by Johnson, et al, (1991) from Earth-based observations |
The maria whose regoliths are of greatest current interest as a source of solar wind volatiles, such as 3He for fusion power, are those that are oldest (most mature) and most titanium-rich. Such maria appear to be most abundant in the vicinity of Tranquillitatis and in Mare Procellarum. In fact, there are indications that mare eruptions over time may have been roughly concentric to this region and contained less and less titanium on the average.- Johnson, et al (1999) have recently published a detailed prediction of He distribution on the Moon based on a new analysis of the Clementine multispectral data sets that estimate global TiO2 distribution and surface maturity (Lucey et al, 1995, 1996, 1998) and the Swindle, et al (1992) solar wind fluence model. The figures below (courtesy of J. R. Johnson) summarizes that recent analysis:
 Clementine Lunar Mosaic
Clementine Lunar Mosaic
 Estimated Helium-3 Abundance
Estimated Helium-3 Abundance
Johnson's analysis is generally consistent with that of Cameron, 1992) based on terrestrial remote sensing data. In the case of Cameron's analysis of Tranquillitatis, Apollo 11 samples tell us that this is a very mature surface so Ti will be a good surrogate for 3He in this region.
 |
Titanium content of soils in Mare Tranquillitatis (after Cameron, 1992). |
 |
Estimated helium resource base in Mare Tranquillitatis (after Cameron, 1992). |
Titanium-rich pyroclastic deposits, such as those on the southwestern rim
of Serenitatis, also may be of interest as a source of solar wind volatiles.
Their regolith should be much more easily mined due to the absence of significant
hard rock, may be more easily processed due to their glassy and fine-grained
nature, and they may be a source of several metal and halogen by-products.
Hydrogen, water, oxygen:
Hydrogen does not appear to vary with Ti abundance in the lunar soil and in mature lunar soils, with minimum measurement on Apollo samples ranging from about 106 ppm to about 17 ppm in the mare, with a mean value of 46 +/- 22 ppm, and up to 146 ppm in the highlands (Fegley and Swindle, 1993, and Heiken, et al, 1991). In fact, recent Lunar Prospector data strongly suggest an inverse relationship between H and Ti, possibly because of hydrogen's affinity for plagioclase feldspar over Fe, Mg, and Ti-rich minerals in contrast to 3He's affinity for the Ti-rich mineral, ilmenite.
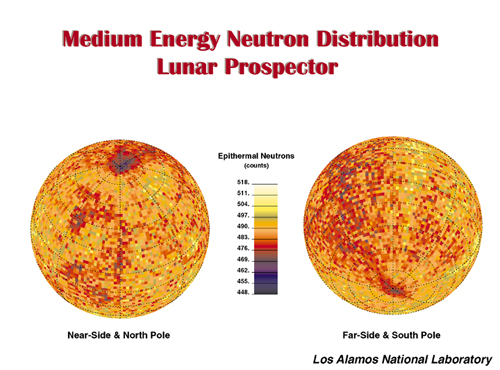
Hydrogen in the lunar soil, in concert with the oxygen in the various minerals, is roughly equivalent to 0.5 liter of water per cubic meter or, to a depth of about two meters, about 106 gallons per square mile. The possibility of water ice at the lunar poles not with standing, lunar settles can make water anywhere.
About 90% of the solar wind hydrogen, a significant portion of which combines with oxygen in the silicates and oxides to form water, can be released from lunar soil by simple heating to about 800o C (Pepin, et al, 1970).
Interplanetary Dust
One other item of interest is the estimate that interplanetary dust particles fall on the earth at an estimated average rate of 30,000-40,000 tons/year (reported in EOS, 1998, 79, 46,p 558). A proportionate amount must also fall on the surface of the Moon and contributes both to the chemistry of the regolith and the texturing of its uppermost surface.
Text:
- Evaluation
of the Regolith of Mare Tranquillitatis as a Source of Volatile Elements
- Cameron (1993) (first few pages)
Helium Resources of Mare Tranquillitatis -- Cameron (1992), pp. 16-38
Dark Mantle Material as a Source of Helium (Cameron)
Questions:
Indigenous
1. Taking the composition of any Apollo 11 or 17 Ti-rich basalt flow, describe possible mineral enrichments and their layered sequence due to slow cooling and gravitational differentiation in an appropriately thick flow.
2. Do modern applications of rare earth elements suggest that it might be worth while to seek concentrations of such elements for use on the Moon or in Space rather than continue to depend on terrestrial sources? Explain in some detail.
3. List and provide summary descriptions of potential additives necessary to sustain lunar regolith and/or hydroponic based agriculture.
Solar Wind
1. Discuss the argument for and against the existence of water ice in the permanently shadowed polar regions of the moon, including comparisons with the other, relatively cold terrestrial planets, i.e., Mercury, Earth, and Mars.
2. What are the considerations that must be taken into account if one were to calculate the modern day steady state concentration of 3He in the upper one centimeter of the lunar regolith?
3. How are estimates made of titanium distribution on the Moon using remotely sensed spectral data from the Earth and from the Clementine spacecraft sensors? (Consider starting with: Clementine and Johnson, 1991))
References Indigenous:
Alllen, C.C., Morris, R.V., and McKay, D.S., 1996 Oxygen Extraction from Lunar Soils and Pyroclastic Glass, Journal of Geophysical Research, 101, 26,085-26,095.
Criswell, D.R., 1996, Lunar Solar Power System: Review of the technology base of an operational LSP System, 47th International Astronautical Congress, October 7-11, Beijing (IAF-96-R.2.04
Desai, C.S., et al, 1993, Development and Mechanical Properties of Structural Materials from Lunar Simulants, in Lewis, J., Matthews, M.S., and Guerrieri, M.L., 1993, Editors, Resources of Near-Earth Space, University of Arizona Press.
Haskin, L.A., et al, 1993, A Geochemical Assessment of Possible Lunar Ore Formation, in Lewis, J., Matthews, M.S., and Guerrieri, M.L., 1993, Editors, Resources of Near-Earth Space, University of Arizona Press.
Heiken, G., et al, 1991, Lunar Source Book, Cambridge University Press, Cambridge, 736p.
Lewis, J., Matthews, M.S., and Guerrieri, M.L., 1993, Editors, Resources of Near-Earth Space, University of Arizona Press.
Sullivan, et al., 1991, Using Space Resources, NASA Johnson Space Center, 27p.
Taylor and Haskin
References Solar Wind:
Arnold, J.R., 1979, Ice on the Moon, Journal of Geophysical Research, v 84, 5659-5668.
Cameron, E.N., 1988, Dark Mantle Material as a Source of Helium, unpublished memorandum to G.L. Kulcinski, WCSAR-TR-AR3-8810-3.
Cameron, E.N., 1992, Helium Resources of Mare Tranquillitatis, Technical Report, WCSAR-TR-AR3-9207-1.
Cameron, E.N., 1993, Evaluation of the Regolith of Mare Tranquillitatis as a Source of Volatile Elements , Technical Report, WCSAR-TR-AR3-9301-1
Fegley, B., Jr., and Swindle, T.D., 1993, Lunar Volatiles: Implications for Lunar Resource Utilization, in Lewis, J., Matthews, M.S., and Guerrieri, M.L., 1993, Editors,Resources of Near-Earth Space, University of Arizona Press.
Heiken, G., et al, 1991, Lunar Source Book, Cambridge University Press, Cambridge, 736p.
Johnson, J.R., et al, 1991, Journal of Geophysical Research, v 96(E3), 18861.
Johnson, J.R., Swindele, T.D., and Lucey, P.G., 1999, Estimated Solar Wind-Implanted Helium-3 Distribution on the Moon, Geophysical Research Letter, 26, 3, 385-388.
Lewis, J., Matthews, M.S., and Guerrieri, M.L., 1993, Editors,Resources of Near-Earth Space, University of Arizona Press.
Lucey, P.G., et al, 1995 Abundance and Distribution of Iron on the Moon, Science, 268, 1150-1153.
Lucey, P.G., et al, 1996 Lunar Titanium Content from UV-VIS Measurements, Lunar and Planetary Science Conference XXVII, 781-782.
Lucey, P.G. et al, 1998 Mapping the FeO and TiO2 Content of the Lunar Surface with Multispectral Imagery, Journal of Geophysical Research, 103, 3679-3699.
Nozette, S. et al, 1996, The Clementine Bistatic Radar Experiment, Science, v 274, 1495-1498.
Pepin, R.O.,et al, 1970, Rare gases in Apollo 11 lunar material, Proceedings of the Apollo 11 Lunar Science Conference, 1443-1454.
Stacy, N.J.S., et al, 1997, Arecibo Radar Mapping of the Lunar Poles: a Search for Ice Deposits, Science, v 276, 1527-1530.
Taylor, L.A., 1993, Evidence of Helium-3 on the Moon: Model Assumptions and Abundances, Proceedings of the Second Wisconsin Symposium on Helium-3 and Fusion Power, WCSAR-TR-AR3-9307-3, 49-56.
Watson, K., et al, 1975, The Behavior of Volatiles on the Lunar Surface, The Moon, v 13, 121.
Text:
Sullivan, et al., 1991, Using Space Resources, NASA Johnson Space Center, 27p.
NEEP533 Syllabus
 |
|
University of Wisconsin Fusion Technology Institute · 439 Engineering Research Building · 1500 Engineering Drive · Madison WI 53706-1609 · Telephone: (608) 263-2352 · Fax: (608) 263-4499 · Email: fti@engr.wisc.edu |
Copyright © 2003 The Board of
Regents of the University of Wisconsin System.
For feedback or accessibility issues, contact
web@fti.neep.wisc.edu.
|