NEEP602 Course Notes (Fall 1996)
Resources from Space
Extraction of Solar Wind Volatiles
Lecture 18
Professor G. L. Kulcinski
March 1, 1996
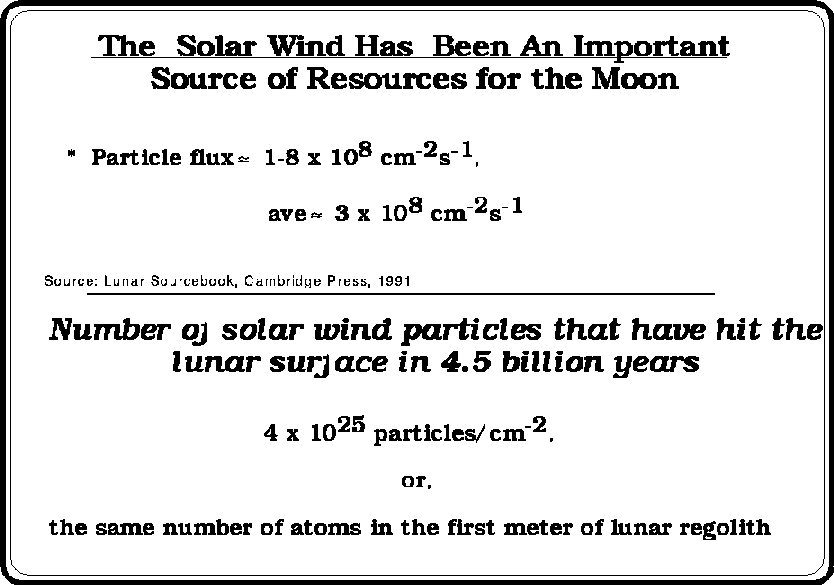
The Solar Wind Volatiles (SWV's) have been "blowing" on the planets (and Moons) of our solar system for some 4.5 billion years. The Solar wind is ionized and therefore is deflected by the Earth's magnetic field.
As the Moon passes in and out of the Solar Wind, and as a consequence of having one side always facing the Earth, the Solar Wind is distributed preferentially on the "far side" of the Moon. The "near side" collects only ~ 1/3 that of the "far side".
In order to calculate the amount of SWV's available for use, we need to know 5 important pieces of information.
A few facts about the Solar Wind are important to our understanding with respect to SWV resources.
The measured concentration of the Solar Wind, and other, volatiles ranges from mass parts per billion to a few tenths of a percent.
Even though these concentrations are small, the total amount of SWV's can be very large, e.g., in the millions to billions of metric tonnes
As an example of what one can obtain from the SWV's in 1 cubic meter of regolith consider the following:
Lunar volatiles have many uses.
In addition, the production of hydrogen and oxygen can be important for future space travel in the Solar System as well as for life support in LEO.
The growing of plants or the life support for humans has some upper and lower limits with respect to gaseous elements or molecules.
The support of humans in space, with efficient recycle of critical materials could require as little as 700-800 kg/y.
The current cost to bring even 700 kg/person-y to the Moon would be enormous.
The location of some SWV's may be augmented by their association with elements that can be detected optically. For example, high titanium containing ilmenite may specifically trap helium isotopes. Therefore, if one knows where Ti is, then it may be a good indication of where the Helium is also. This association was made by Professor E. N. Cameron (1988, 1992, 1993) of the University of Wisconsin.
It is clear that the high Ti regions of the Moon are in the Mare regions. The concentration of the He is much higher in those regions.
The regolith in those regions is made up of very fine grains which has been gardened by meteorites over billions of years
The depth of the regolith varies from site to site and may range from 3 to 12 meters.
Because of the constant meteorite bombardment, the regolith has been pulverized to a very small grain size, i. e., to between 40-130 microns mean diameter.
The solar wind, at 1 keV/amu, has a relatively short range in the regolith. It has been calculated, by K. Kulhman (1996), that the range of a 1 keV proton is ~ 140 Å in ilmenite while a 4 keV 4He ion will penetrate to a depth of ~320 Å.
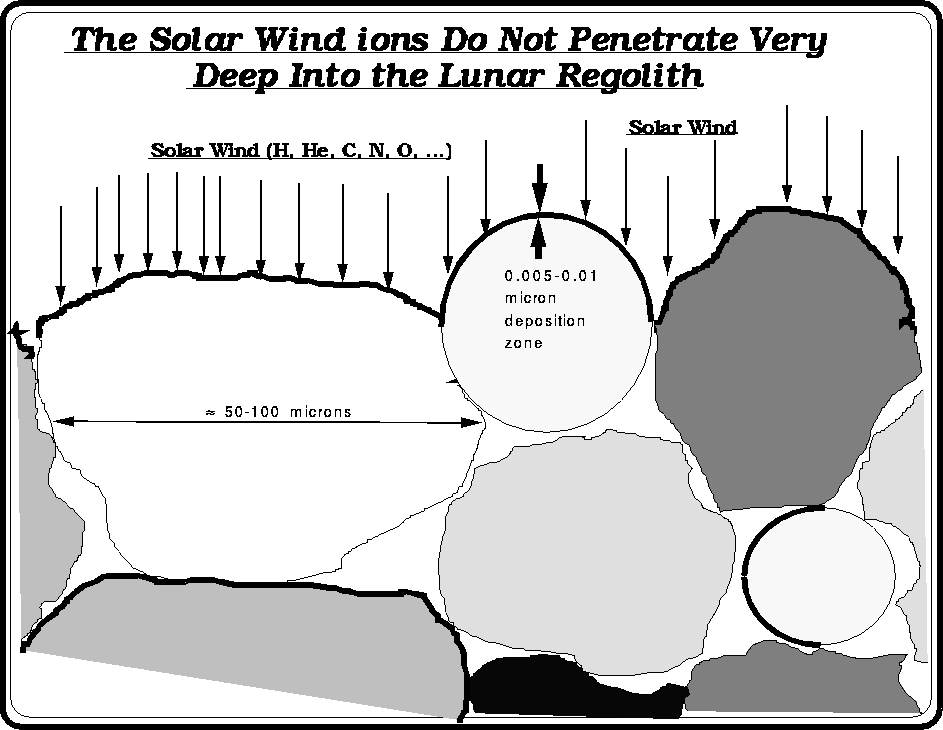
Since the Solar Wind does not penetrate very deep into the ilmenite grains, the SWV's reside mainly in the near surface region. The "gardening" process can result in SW implanted grains that exist far below the surface of the regolith.
The efficiency of SWV evolution depends inversely on the size of the regolith particle.
The concentration of the SWV, 3He, is relatively constant with depth, at least down to 2.4 meters.
The concentration of the SWV, 3He, in lunar regolith follows the 1/r law predicted earlier.
Data from Eberhart (1970), Kirsten (1970), and Hintenberger (1970)
Cameron (1988) has confirmed that most of the SWV, 3He, is contained in particles smaller than 50 microns.
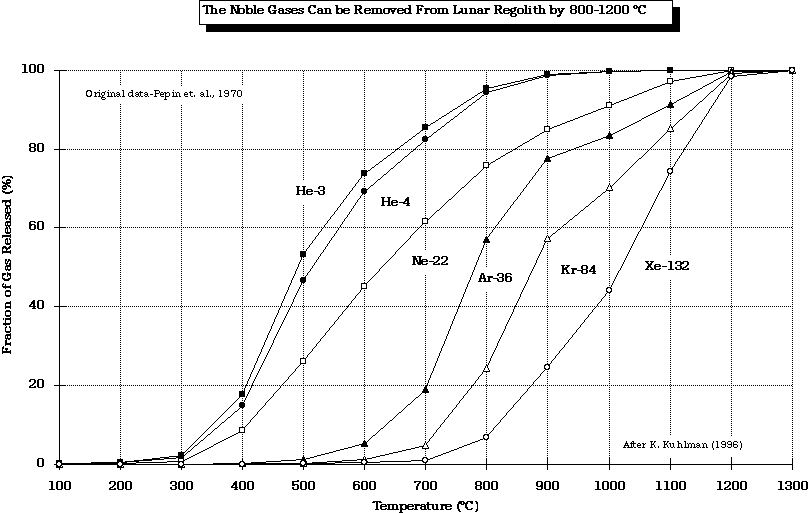
As lunar regolith is heated, a very complex SWV release pattern is evident.
The release of the noble gases (He, Ne, Ar, Kr, and Xe) from lunar regolith reveals that progressively higher temperatures are required.
Original data from Pepin (1970) modified by Kuhlman (1996)
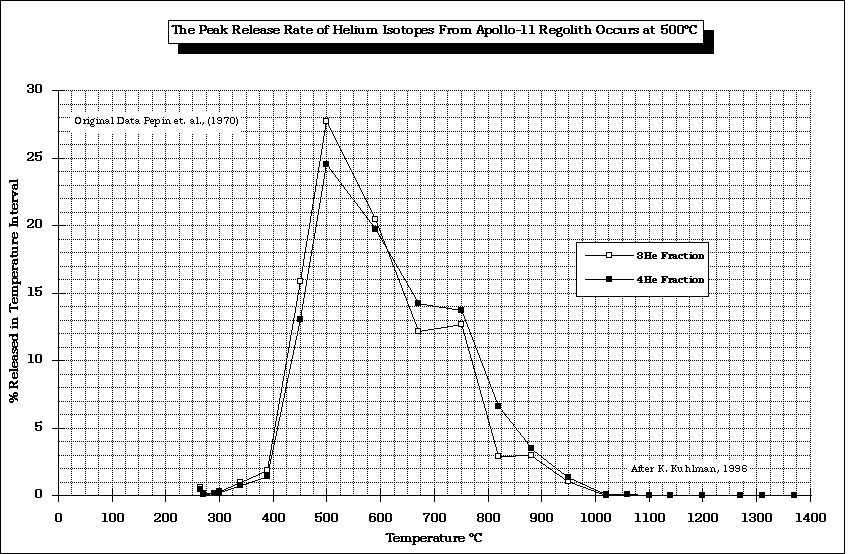
The peak release temperature for He from Apollo-11 regolith occurs at 500 oC.
Original data from Pepin (1970) modified by Kuhlman (1996)
The conclusion of the data presented is that there are many trade-offs to be considered before one picks a temperature at which the SWV of choice is recovered. For the case of He, the maximum should be below 700-800 oC because of the release of S compounds which may compromise the integrity of the mining equipment.
References
Bula, R. J., Wittenberg, L. J., Tibbets, T. W., and Kulcinski, G. L., 1988, "Potential of Derived Lunar Volatiles for Life Support", p. 547 in The Second Conference on Lunar Bases and Space Activities of the 21st Century, W. W. Mendell ed., NASA Conference Publication 3166, Vol. 1
Cameron, E. N., 1988, "Helium Mining on the Moon: Site Selection and Evaluation", University of Wisconsin Technical Report, WCSAR-TR-AR3-8810-6.
Cameron, E. N., 1992, "Helium Resources of Mare Tranqillitatis", University of Wisconsin Technical Report, WCSAR-TR-AR3-9207-1.
Cameron, E. N., 1993, "Evaluation of the Regolith of Mare Tranqillitatis as a Source of Volatile Elements," University of Wisconsin Technical Report, WCSAR-TR-AR3-9301-1
Carrier, W. D. III, Mitchell, J. K., 1989
Eberhardt, P., et. al., 1970, "Trapped Solar Wind Noble Gases, Exposure Age and K/Ar-age in Apollo-11 Lunar Fine Material", Proc. Apollo-11 Lunar Conf. 2:1037-1070.
Fegley, B., Jr., and Swindle, T. D., 1993, "Lunar Volatiles: Implications for Lunar Resource Utilization", in Lewis, J., Matthews, M.S., and Guerrieri, M. L., 1993, Editors, Resources of Near-Earth Space, University of Arizona Press.
Gibson, E. K. Jr., and Johnson, F. S., 1971, Proceedings 2nd Lunar Sci. Conf., Vol. 2, p. 1351.
Haskin, L., 1988, "Water and Cheese From the Lunar Desert: Abundances and Accessibility of H, C, and N on the Moon", P. 393 in The Second Conference on Lunar Bases and Space Activities of the 21st Century, W. W. Mendell ed., NASA Conference Publication 3166, Vol. 1
Heiken, G., et al, 1991, Lunar Source Book, Cambridge University Press, Cambridge, 736p.
Hintenberger, H., et. al., 1970, "Concentrations and Isotopic Abundances of the Rare Gases, Hydrogen and Nitrogen in Apollo-11 Lunar Matter", Proc. Apollo-11 Lunar Conf. 2:1607-1625.
Horz, F., Gibbons, R. V., Gault, D. E., Hartung, J. B., and Brownlee, D. E., 1975, "Some Correlation of Rock Exposure Ages and Regolith Dynamics", Proc. 6th Lunar Sci. Conf., p. 3495-3508.
Kirsten, T., Muller, O., Steinbruhnn, F., and Zahringer, J., 1970, "Study of Distribution and Variations of Rare Gases in Lunar Material by a Microprobe Technique", Proc. Apollo-11 Lunar Conf. 2:1331-1343.
Kuhlman, K., 1996, unpublished data.
Pepin, R. O., Nyquist, L. E., Phinney, D., and Black, D. C., 1970, "Rare Gases in Apollo 11 Lunar Material", Proc. Apollo 11 Lunar Sci. Conf., pp. 1443-1454
Swindle, T. D., Glass, C. E., and Poulton, M. M., 1990, "Mining Lunar Soils for 3He", UA/NASA Space Engineering Research Center TM-90/1 (Tucson: UA/NASA SERC).
Taylor, G. J., 1995, Univ. of Hawaii, artwork on the food and soft drinks
Representative Questions
1.) Compare the energy required to extract 1 kg of 3He from lunar regolith at 700 oC to that required at 900 oC. Use the data presented in class for the evolution as a function of temperature and be clear about your assumptions on the thermal properties of lunar regolith
2.) Reproduce L. Haskin's calculation on the amount of food one could produce from the Solar Wind Volatiles in 1 m3 of lunar regolith.
3.) Discuss the engineering considerations that you would have to consider if you wanted to gather the maximum amount of nitrogen from lunar regolith.
 |
|
University of Wisconsin Fusion Technology Institute · 439 Engineering Research Building · 1500 Engineering Drive · Madison WI 53706-1609 · Telephone: (608) 263-2352 · Fax: (608) 263-4499 · Email: fti@engr.wisc.edu |
Copyright © 2003 The Board of
Regents of the University of Wisconsin System.
For feedback or accessibility issues, contact
web@fti.neep.wisc.edu.
|
 Solar Wind
Solar Wind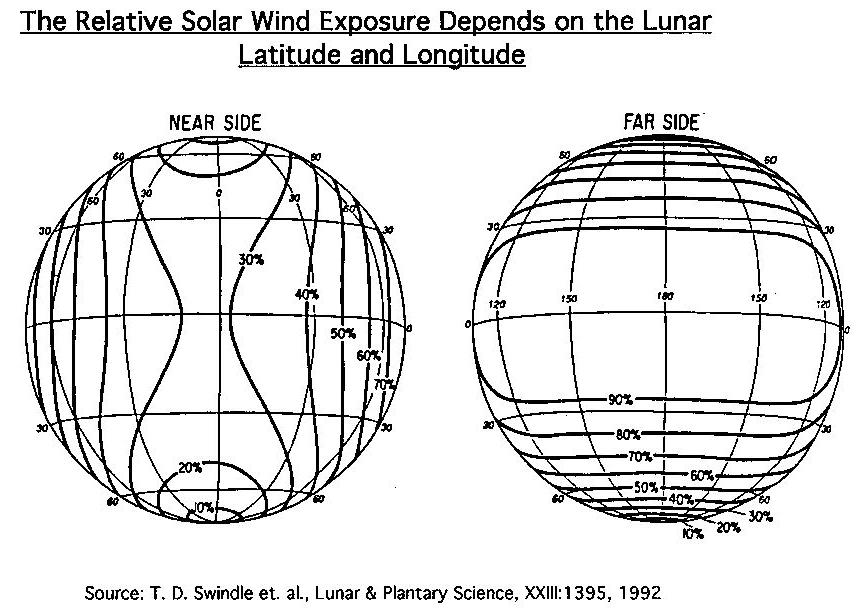 (Swindle, 1992)
(Swindle, 1992)

 (Modified from Fegley and Swindle, 1993)
(Modified from Fegley and Swindle, 1993)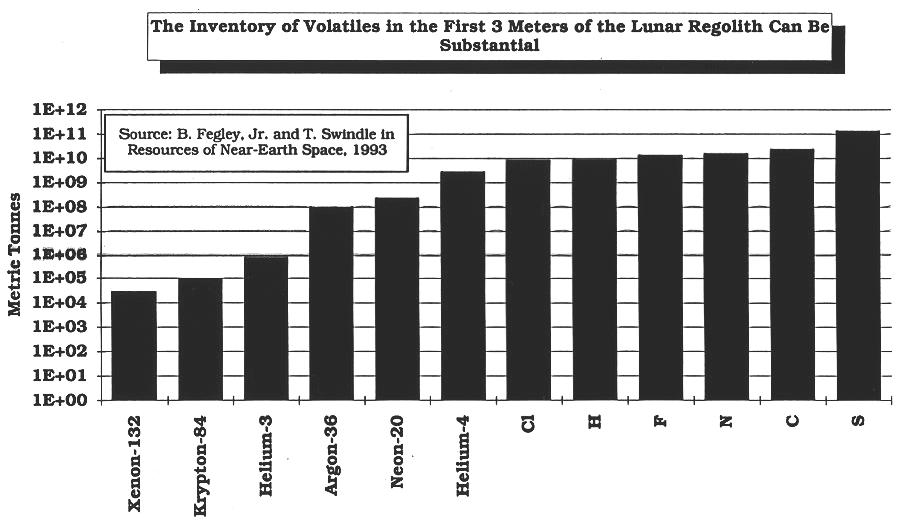 (calculated from data in Fegley and Swindle, 1993)
(calculated from data in Fegley and Swindle, 1993) (Modified from J. Taylor and L Haskin)
(Modified from J. Taylor and L Haskin)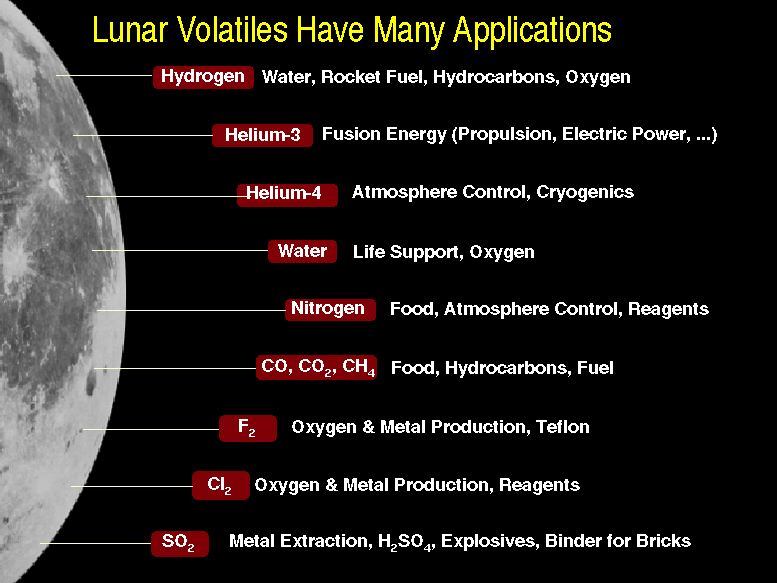
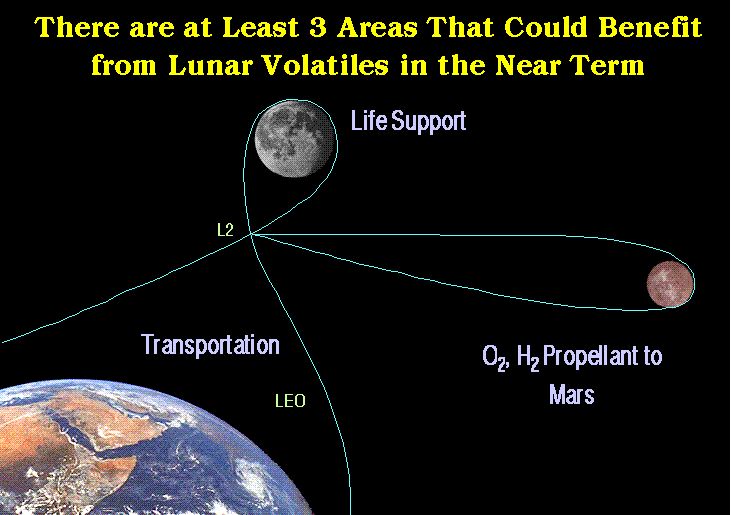
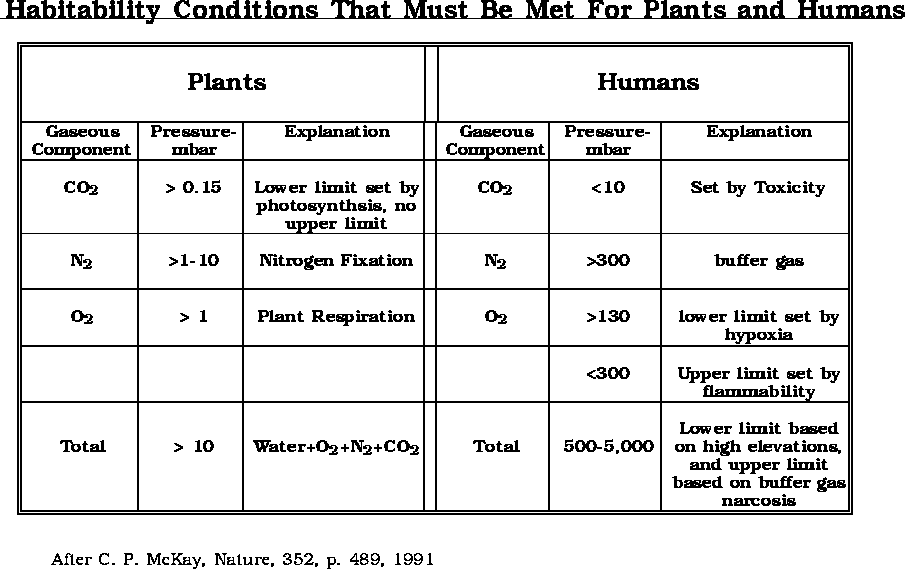
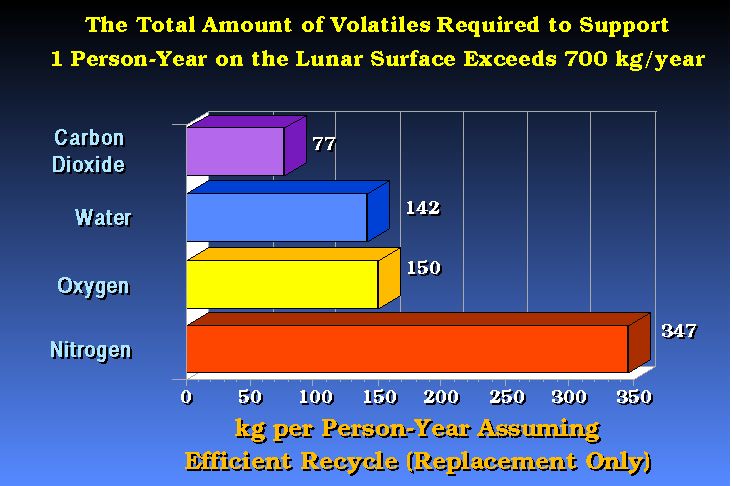
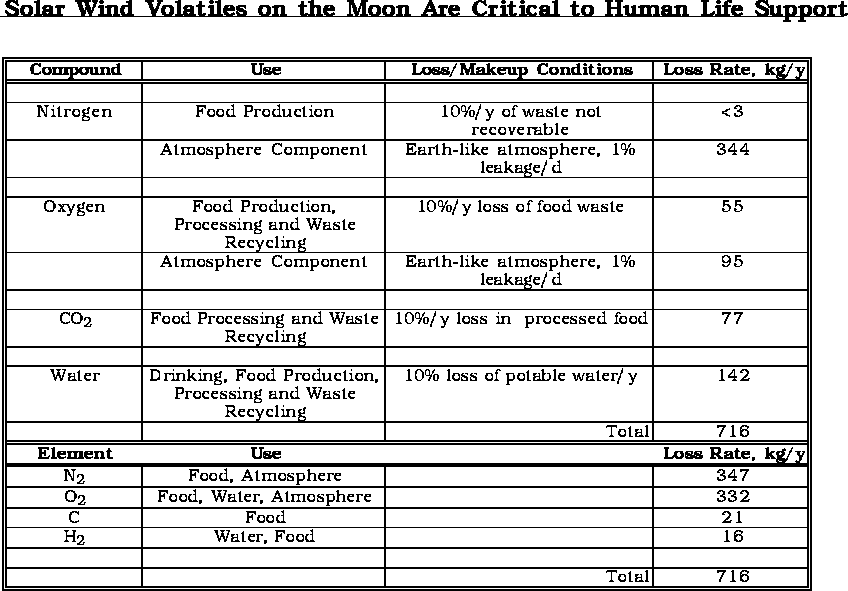 (after Bula et. al., 1988)
(after Bula et. al., 1988)
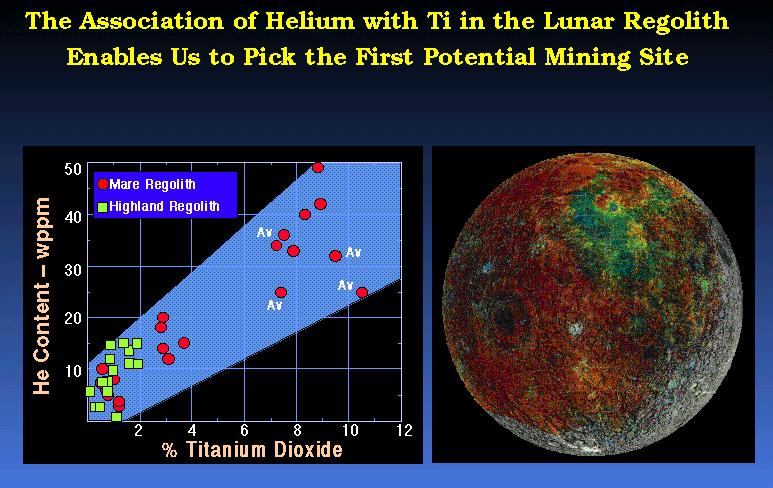

 NASA Photo
NASA Photo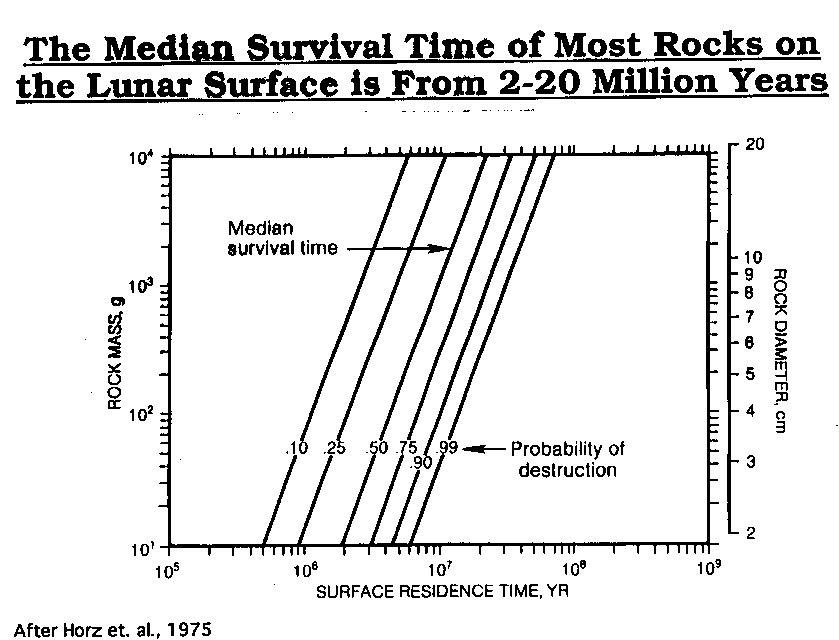 After Horz et. al., 1975
After Horz et. al., 1975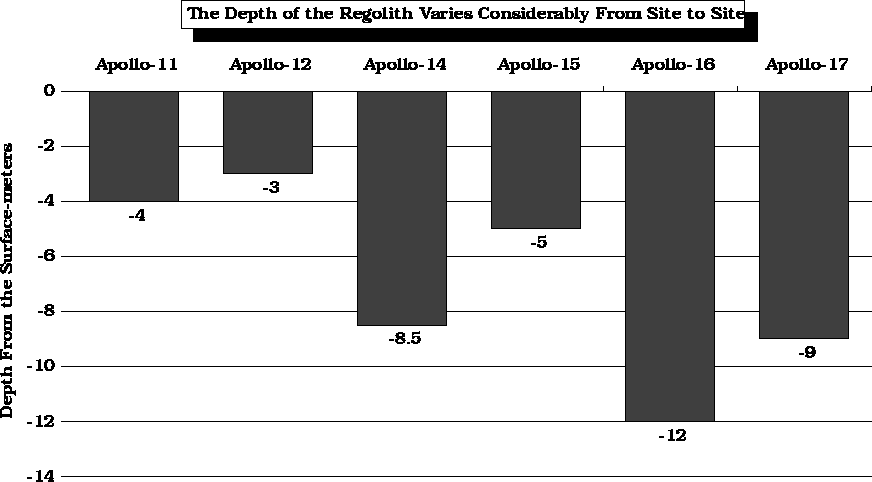
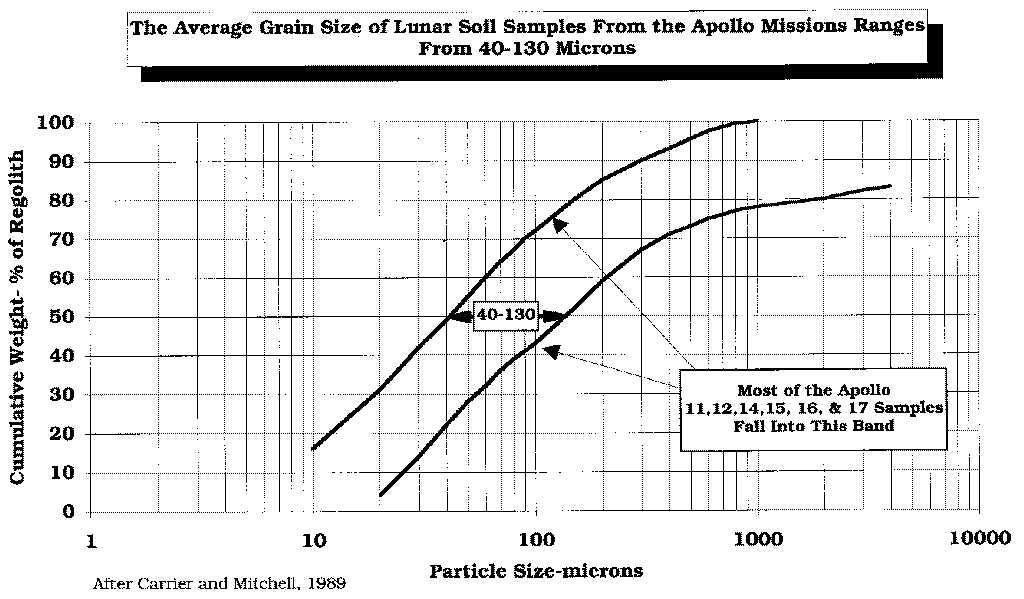 After Carrier and Mitchell, 1989.
After Carrier and Mitchell, 1989.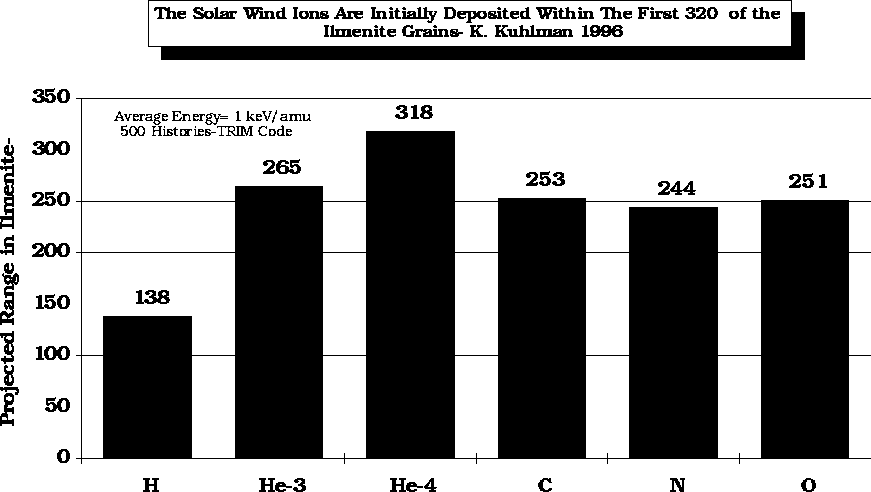
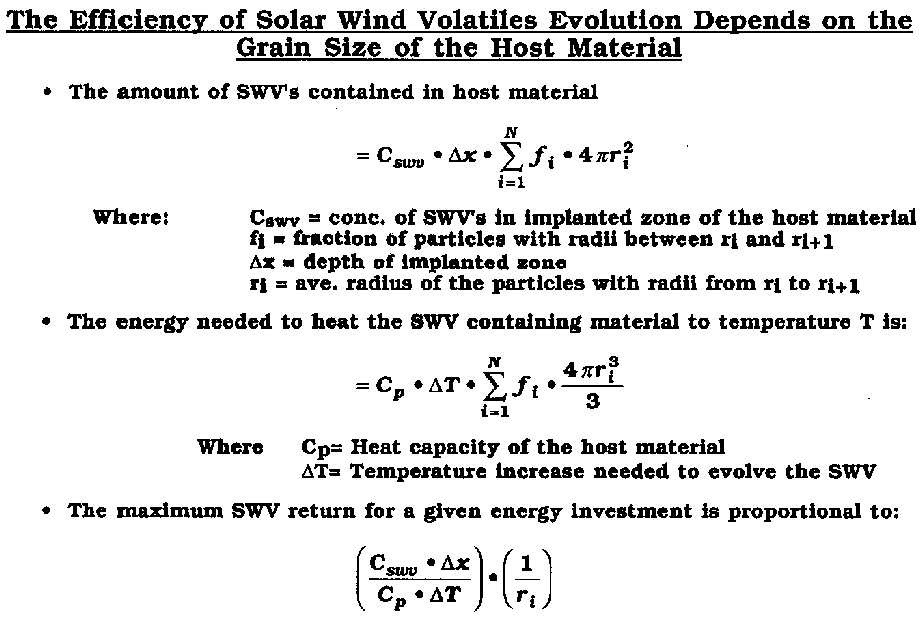
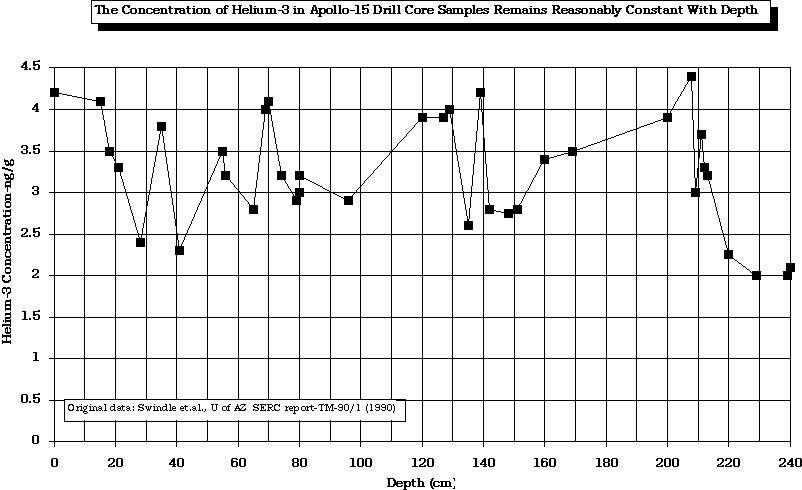 After Swindle (1990)
After Swindle (1990)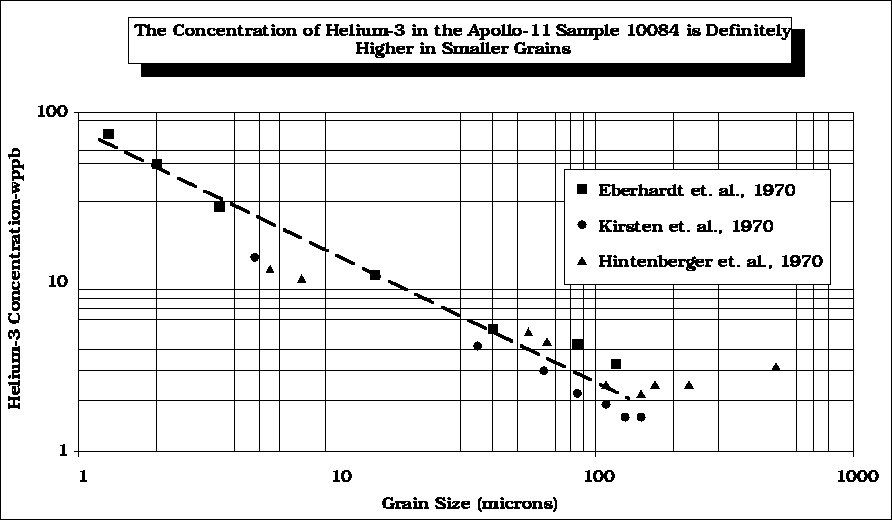
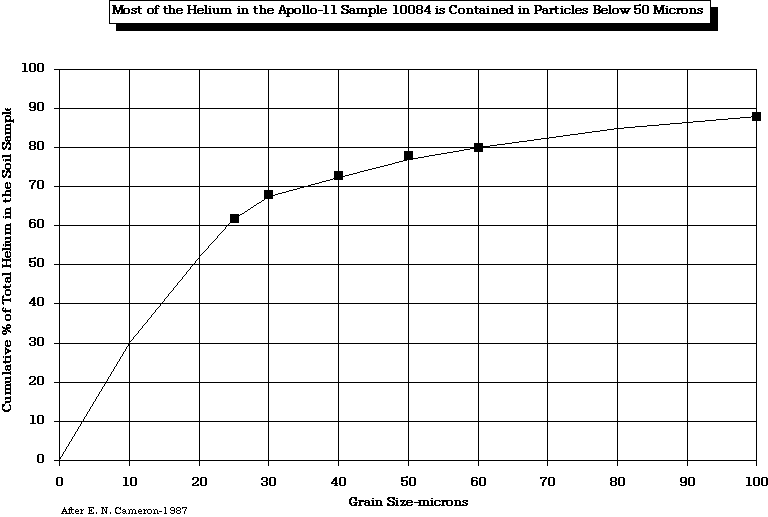
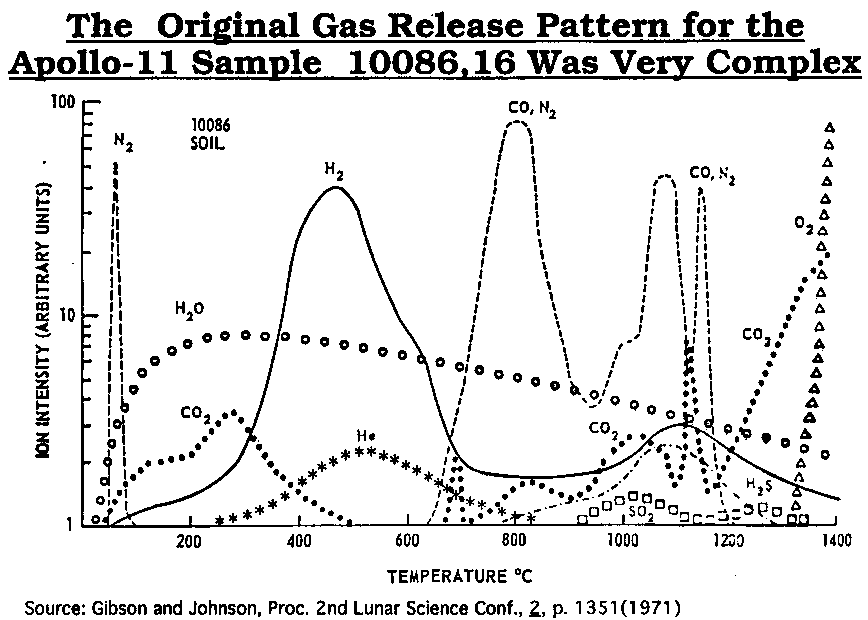 after Gibson and Johnson (1971)
after Gibson and Johnson (1971)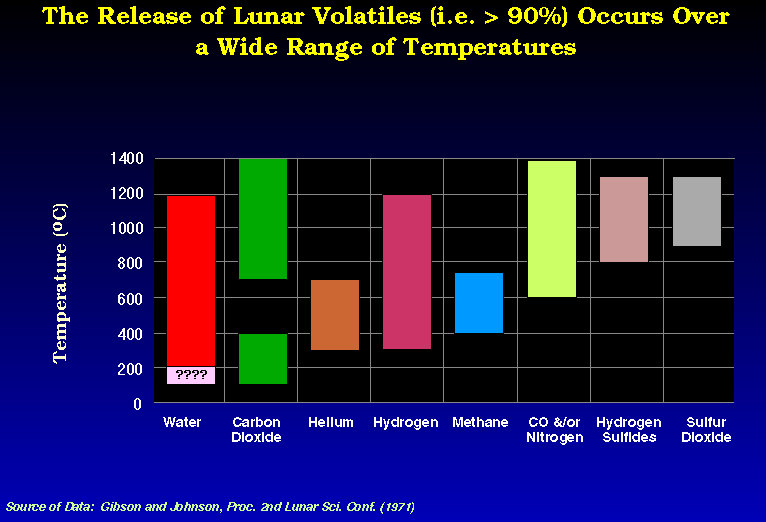 modified from Gibson and Johnson (1971)
modified from Gibson and Johnson (1971)