NEEP602 Course Notes (Fall 1997)
Resources from Space
Extraction Techniques-Oxygen From Lunar Material
Lecture 21
Professor G. L. Kulcinski
October 20, 1997
Oxygen is one of the most valuable resources in space outside of the fusion fuel, 3He. It is used for life support, propulsion and for producing electricity in fuel cells.

An example of how one might use oxygen in conjunction with the extraction of SWV's is shown in the next 3 figures. The basic idea is that instead of removing the hydrogen from the output stream of a lunar miner, one could recycle the hydrogen to increase the water production from ilmenite heated to ~ 700 oC. Once enough water is produced and the oxygen extracted by electrolysis, the fuel cycle loop can be closed and all of the hydrogen generated by the miner can be removed for use elsewhere. See the 3 figures below for more details (Kulcinski et al., 1996).



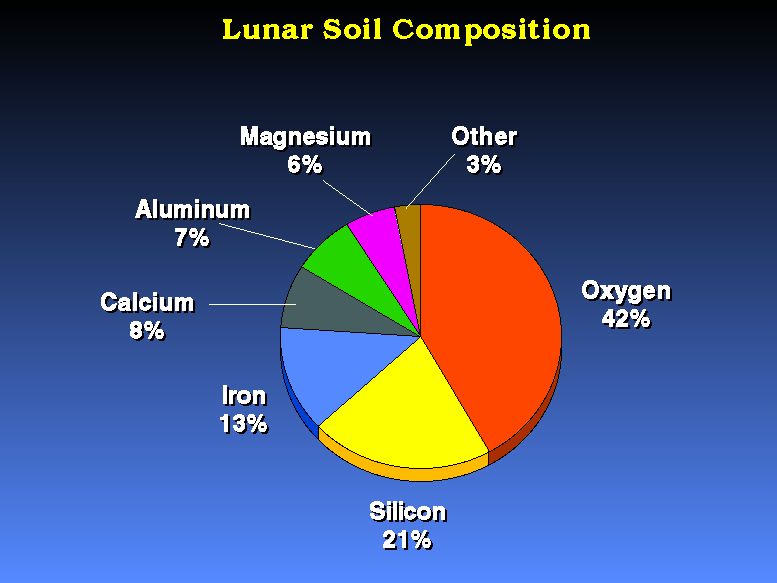
Remember that there is plenty of oxygen on the Moon (e.g., 42% of the Moon is oxygen)
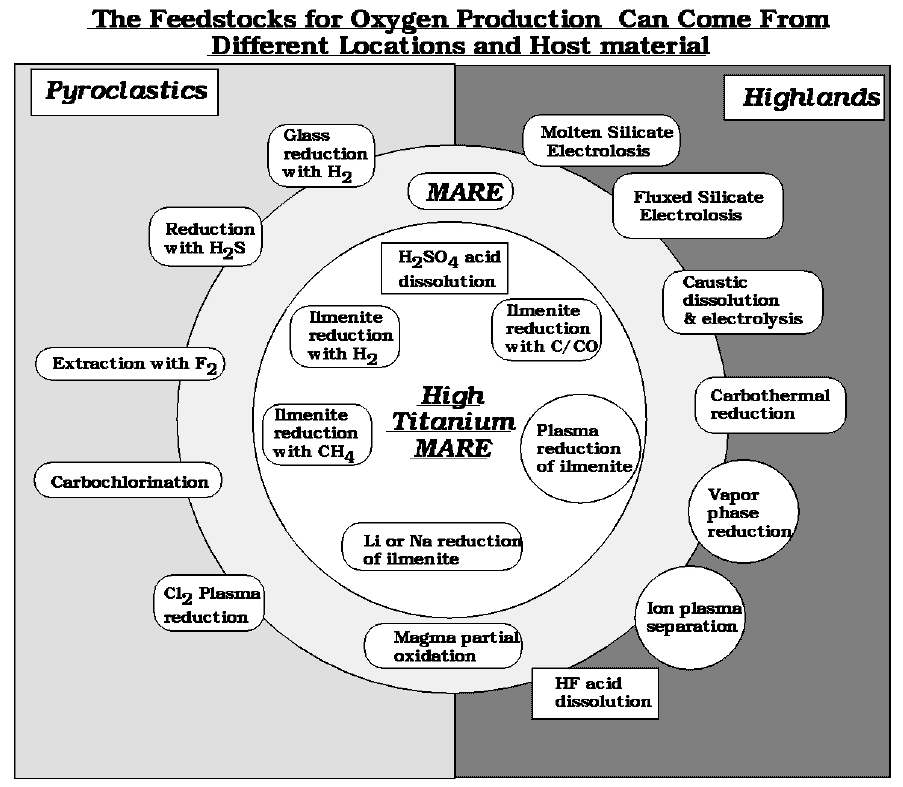
There have been at least 20 different ways proposed and tested experimentally, to produce oxygen from lunar material. See Taylor and Carrier (1993).

Another way to look at this area is with respect to the feed stocks or host material best suited to each of the proposed extraction processes.
The solid/gas reduction concept relies on the chemical removal of oxygen from an oxide. Remember that some oxides, such as CaO, have very strong bonds, while other oxides such as FeO have weak bonds.

The use of hydrogen to reduce ilmenite for the production of lunar oxygen was first proposed in 1979.

The Carbotek Company has patented a process for the extraction of lunar hydrogen (see Gibson and Knudsen, 1990).

The yield of oxygen from lunar soils is strongly dependent on the initial Fe content.

Lunar glass may be one of the best sources of oxygen.
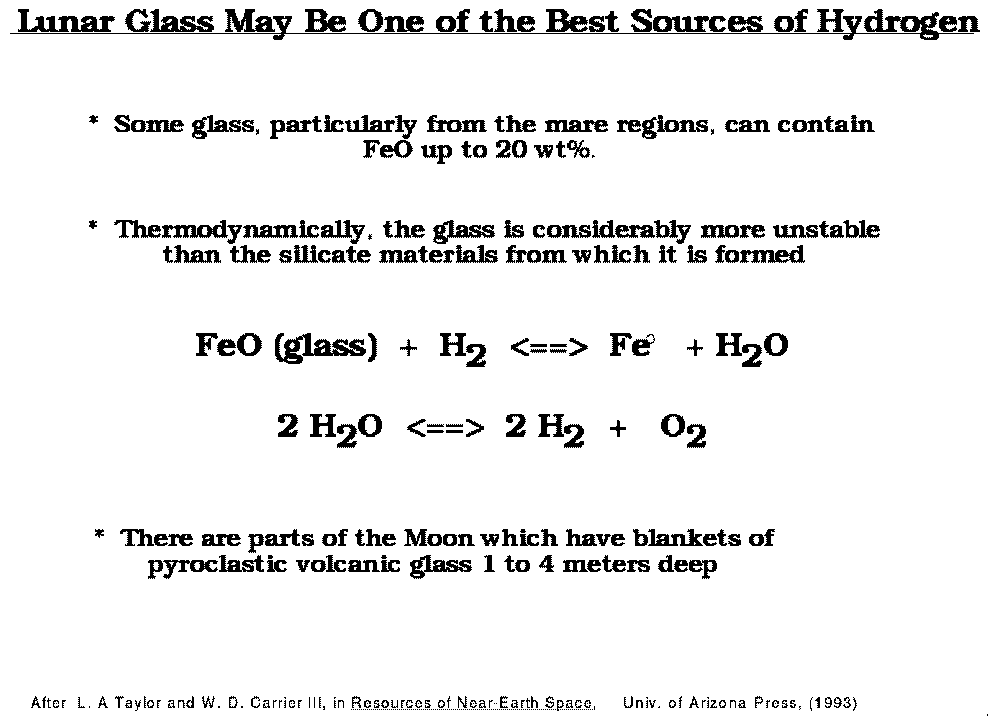
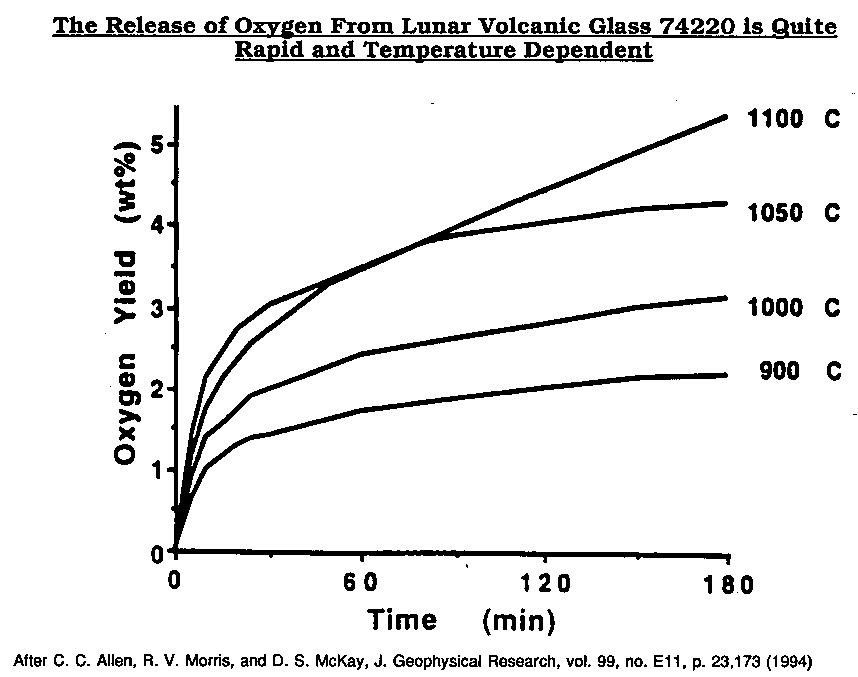
The release of oxygen from lunar volcanic glass is quite rapid and temperature dependent.
Carbon compounds can also be used to extract oxygen from lunar materials.

Another promising method for oxygen extraction is from molten silicates.

There are many other useful products that can be derived from the Molten Silicate Process.

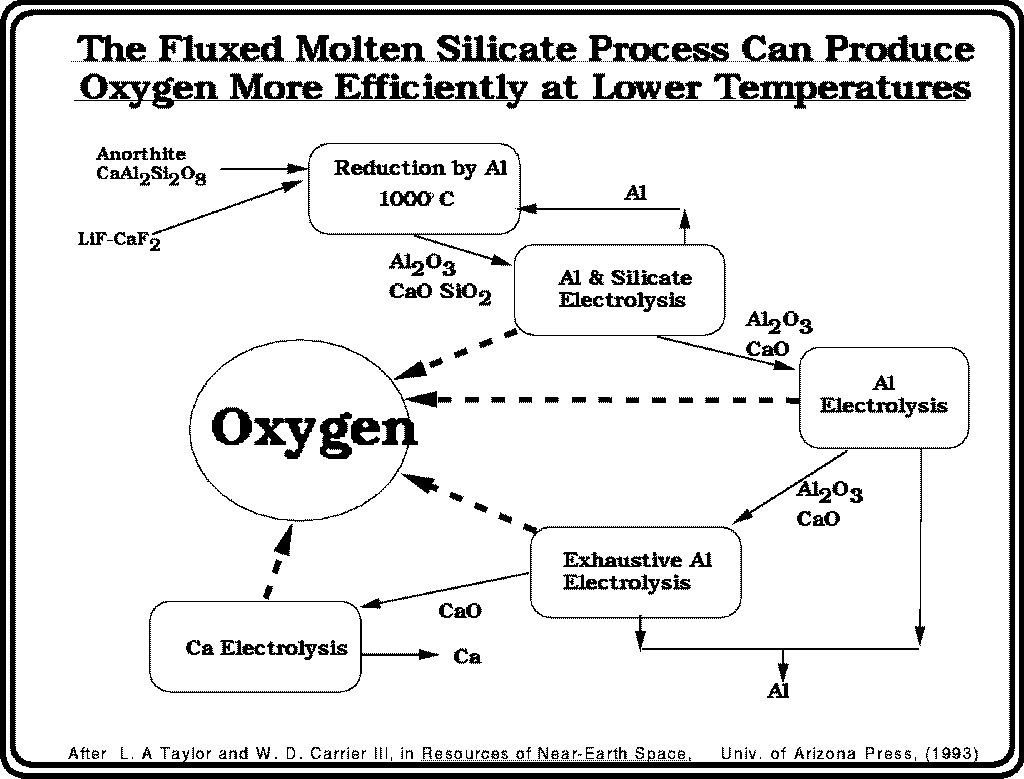
The Fluxed Molten Silicate Process can produce oxygen more efficiently at lower temperatures.
The Vapor Phase reduction process utilizes temperature from 2,000 to 10,000 oK.

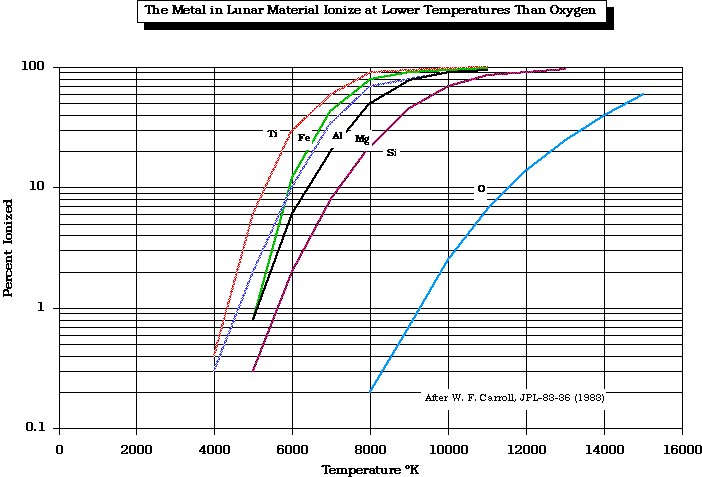
Plasma separation processes rely on the fact that metals remain ionized at lower temperatures than non-metals.

The majority of lunar oxygen producing schemes require between 20-50 kWh/kg of oxygen collected.

The ilmenite-based processes require the highest mass throughput and highest power consumption.

The conclusion that one comes to is that there are many ways to produce oxygen from lunar material. These processes can be tested on Earth and narrowed to a select group for eventual testing on the Moon.
References
Allen, C. C., Morris, R. V., and McKay, D. S., 1994, "Experimental Reduction of Lunar Mare Soil and Volcanic Glass", J. Geophysical. Res., Vol. 99, No. E11, PP. 23,173-23, 185, Nov. 25, 1994
Burt, D. M., 1988, "Lunar Mining of Oxygen Using Fluorine", p. 423 in The Second Conference on Lunar Bases an Space Activities of the 21st Century, ed., W. W. Mendell, NASA Conference Publication 3166, 1988
Carroll, W. F., 1983, "Research on the Use of Space Resources", Jet Propulsion Laboratory Doc. JPL-83-36 (1983)
Gibson, M. A., and Knudsen, C. W., 1990, "Lunar Hydrogen Recovery Process", United States Patent 4,938,946, July 3, 1990.
Kulcinski, G. L., Sviatoslavsky, I. N., and Wittenberg, L. J., 1996, "Impact of Lunar Volatiles Produced During 3He Mining Activities" , Univ. of Wisconsin Report UWFDM-1001, January, 1996.
Mason, L. W., 1992, "Beneficiation and Comminution Circuit for the Production of Lunar Liquid Oxygen (LLOX)", p. 1139 in SPACE '92, eds., W. Z. Sadeh, S. Sture, and R. J. Miller, American Soc. of Civil Engrs., NY, 1992
McKay, D. S., and Allen, C. C., (1996), Hydrogen Reduction of Lunar Materials for Oxygen Extraction on the Moon", Amer. Inst. Aeronautics & Astronautics paper AIAA 96-0488, presented at the 34th Aerospace Sciences Meeting in Reno, NV, Jan. 15-18, 1996.
Taylor , L. A., and Carrier, W. D. III, 1993, "Oxygen Production on the Moon: An Overview and Evaluation", p. 69 in Resources of Near-Earth Space, eds.,
J. Lewis, M. S. Matthews, and M. L. Guerrieri, Univ. of Arizona Press, Tucson, AZ (1993)
Steurer, W. H., 1982, "Extraterrestrial Materials Processing", Jet Propulsion Laboratory Doc. JPL-82-41 (1982)
Williams, R. J., McKay, D. S., Giles, D., and Bunch, T. E., 1979, "Mining and Beneficiation of Lunar Ores", p. 275 in Space Resources and Space Settlements, NASA SP-428
Representative Questions
1.) Why are the methods which use aqueous solutions for extracting oxygen from lunar materials not favored on the Moon?
2.) If the Magma Electrolysis process requires the least amount of energy per kg of oxygen obtained, why is it not the most favored process?
3.) What are the advantages and disadvantages of the plasma pyrolosis process?
 |
|
University of Wisconsin Fusion Technology Institute · 439 Engineering Research Building · 1500 Engineering Drive · Madison WI 53706-1609 · Telephone: (608) 263-2352 · Fax: (608) 263-4499 · Email: fti@engr.wisc.edu |
Copyright © 2003 The Board of
Regents of the University of Wisconsin System.
For feedback or accessibility issues, contact
web@fti.neep.wisc.edu.
|