NEEP602 Course Notes (Fall 1997)
Resources from Space
Extraction of Solar Wind Volatiles
Lecture 19
Professor G. L. Kulcinski
Oct. 15, 1997
- What are the Solar Wind Volatiles (SWV's)?
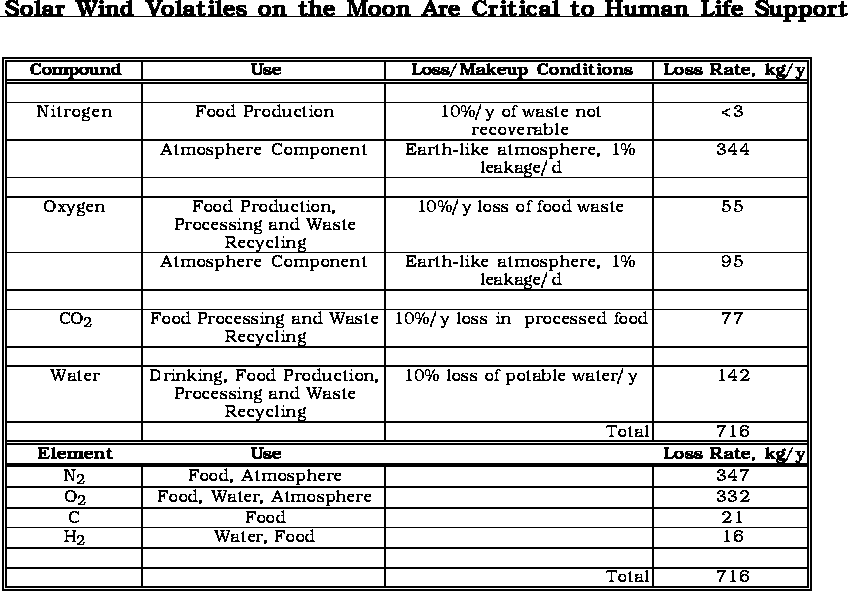
- What are the SWV's good for?
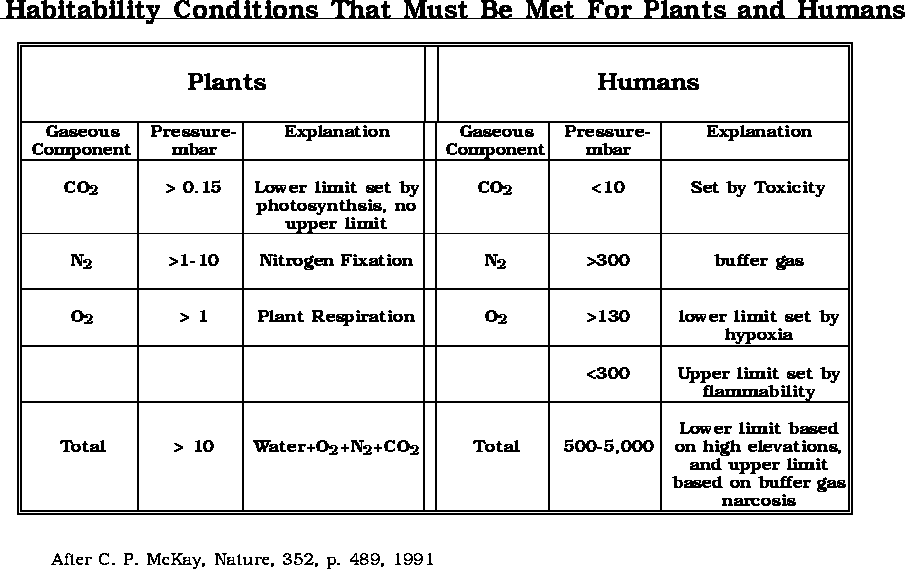
- What is the state of the SWV's on the Moon?
- At what temperature are the SWV's evolved from lunar regolith?
The Solar Wind Volatiles fall into 2 general classes (Lunar Sourcebook, Cambridge, 1991):
1) Biogenic elements (H, C, and N)
2) Noble gases (He, Ne, Ar, Kr, and Xe)
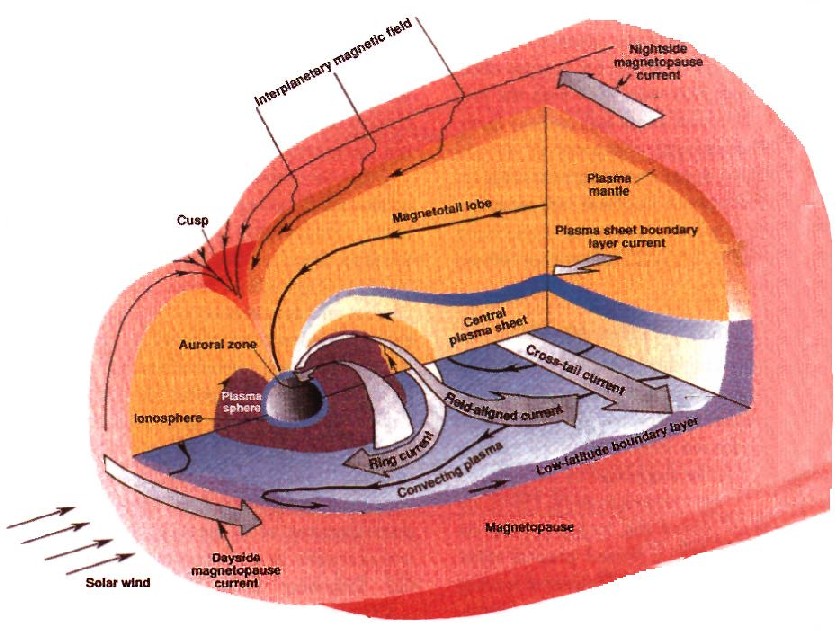 Solar Wind
Solar Wind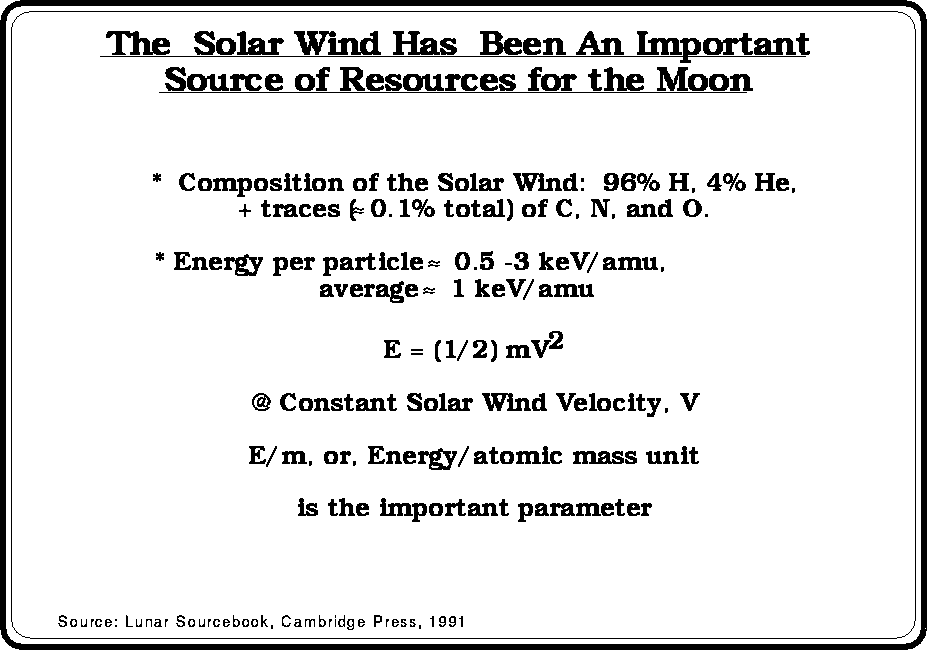

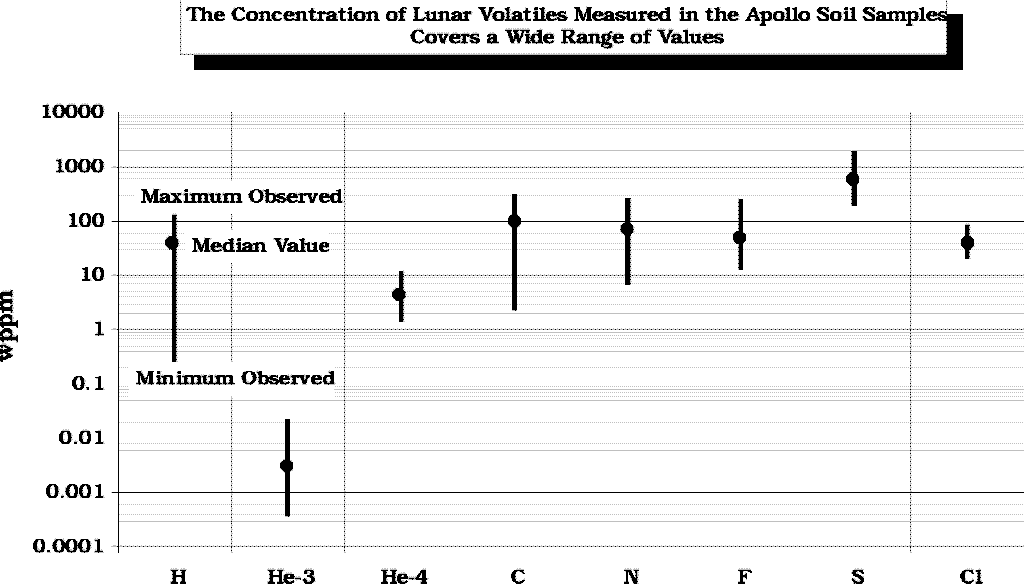 (Modified
from Fegley and Swindle, 1993)
(Modified
from Fegley and Swindle, 1993)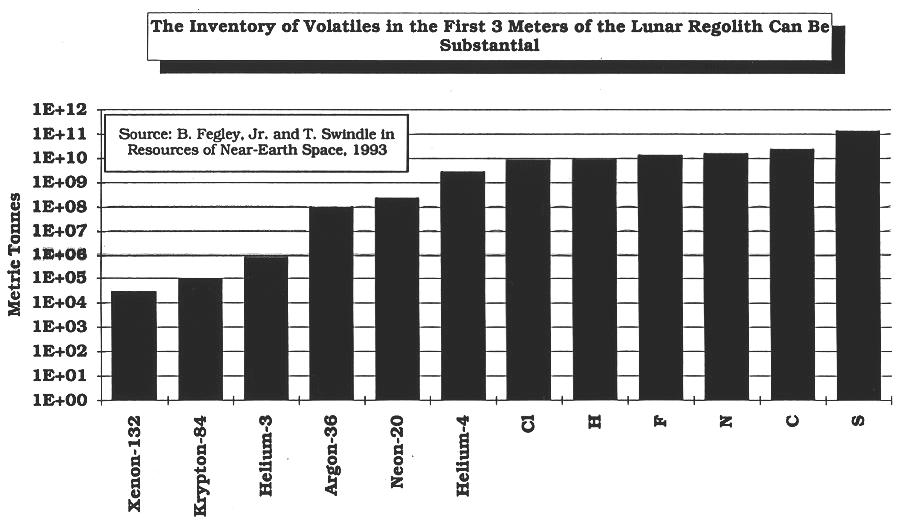 (calculated
from data in Fegley and Swindle, 1993)
(calculated
from data in Fegley and Swindle, 1993) 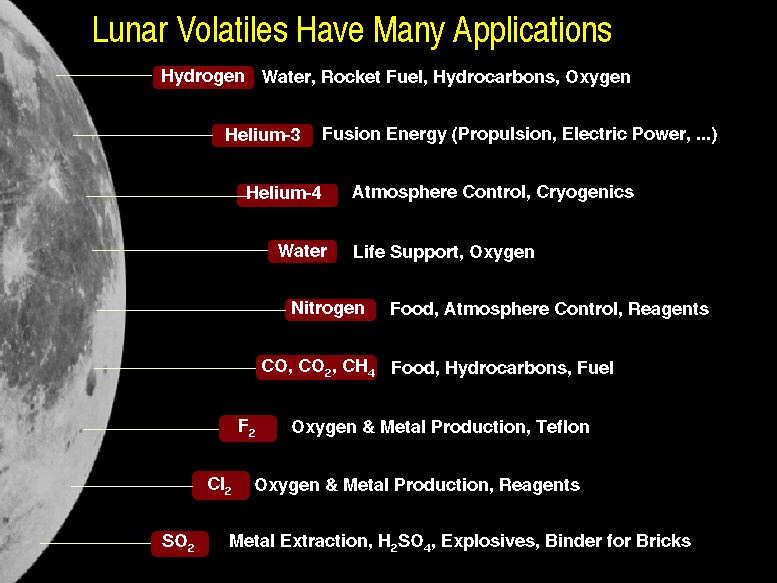
 (after
Bula et. al., 1988)
(after
Bula et. al., 1988)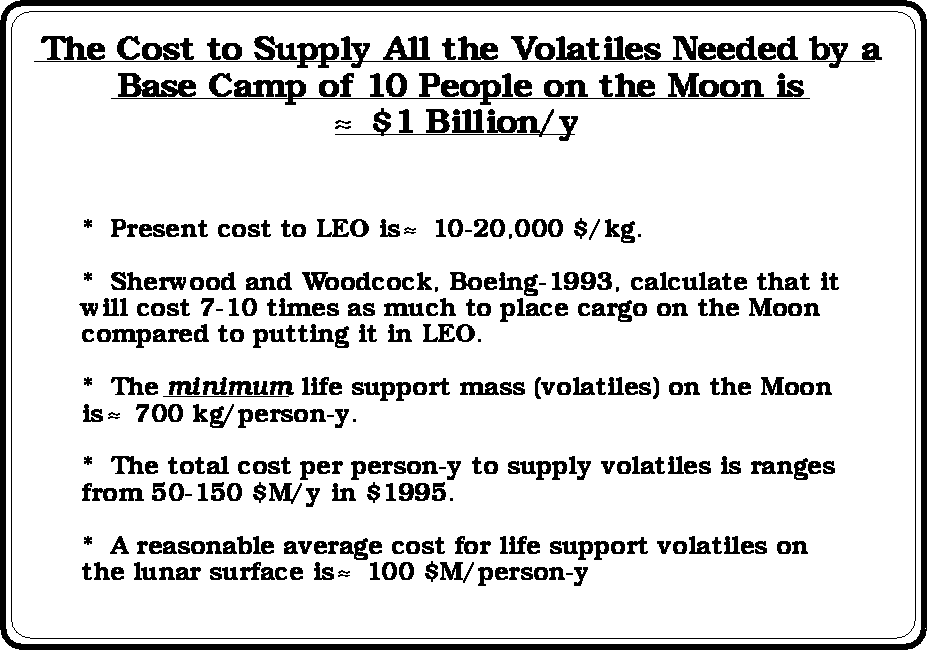
 (Modified
from J. Taylor and L Haskin)
(Modified
from J. Taylor and L Haskin)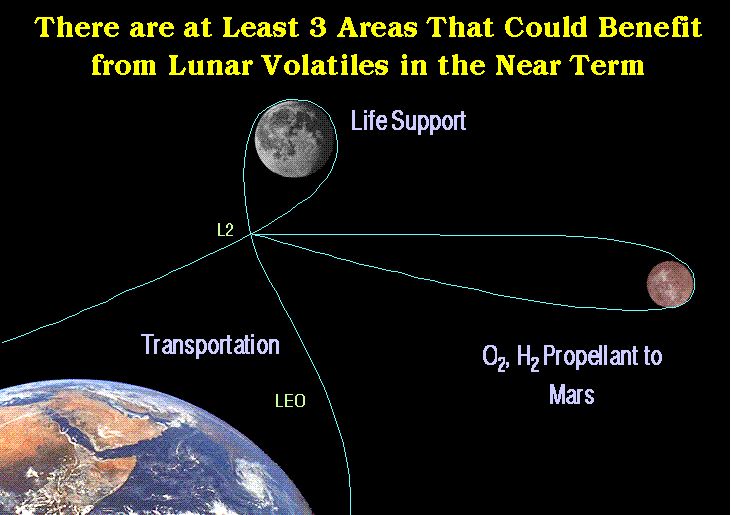

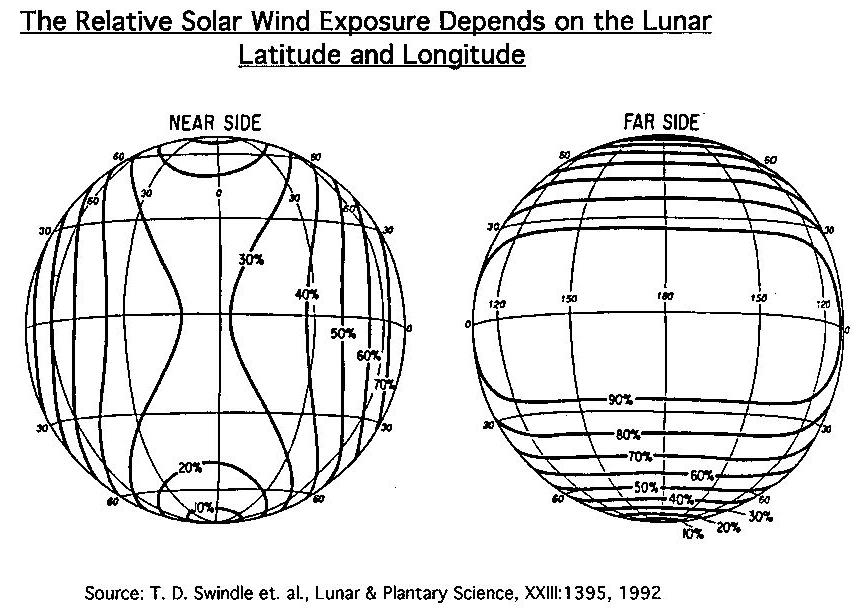 (Swindle,
1992)
(Swindle,
1992)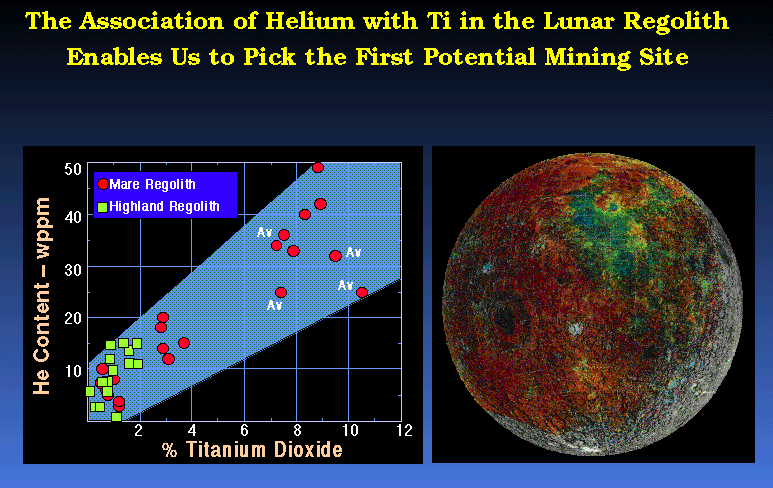
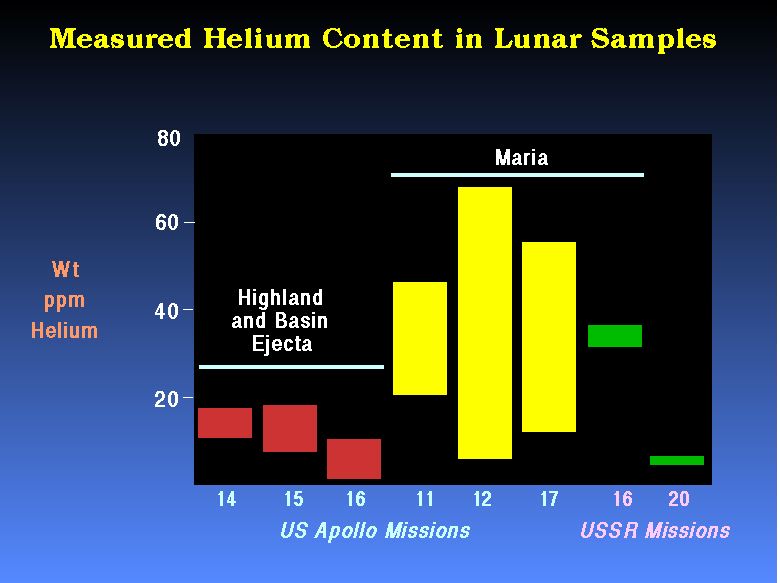
 NASA
Photo
NASA
Photo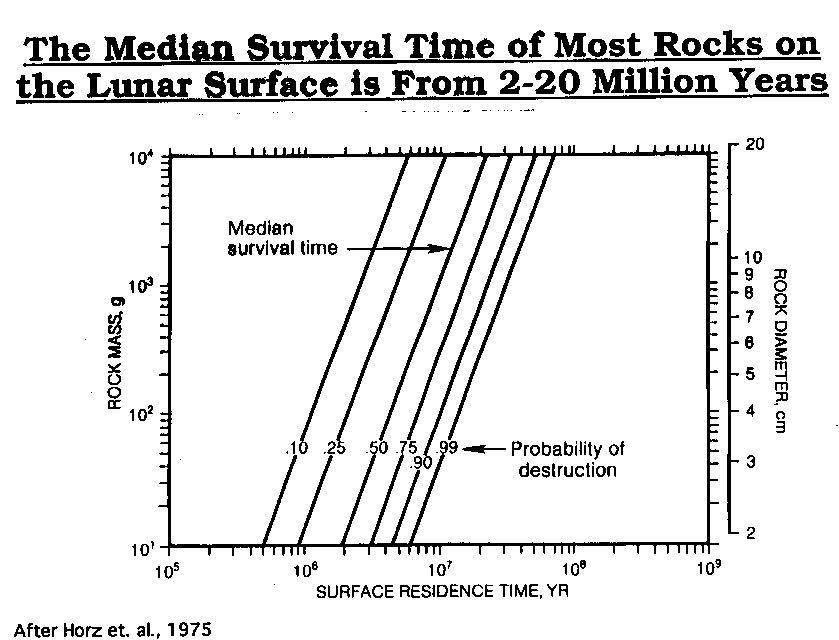 After
Horz et. al., 1975
After
Horz et. al., 1975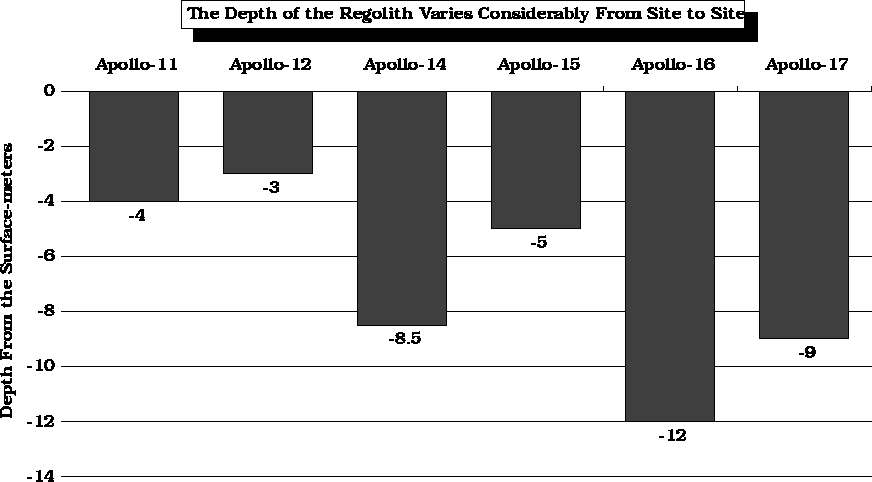
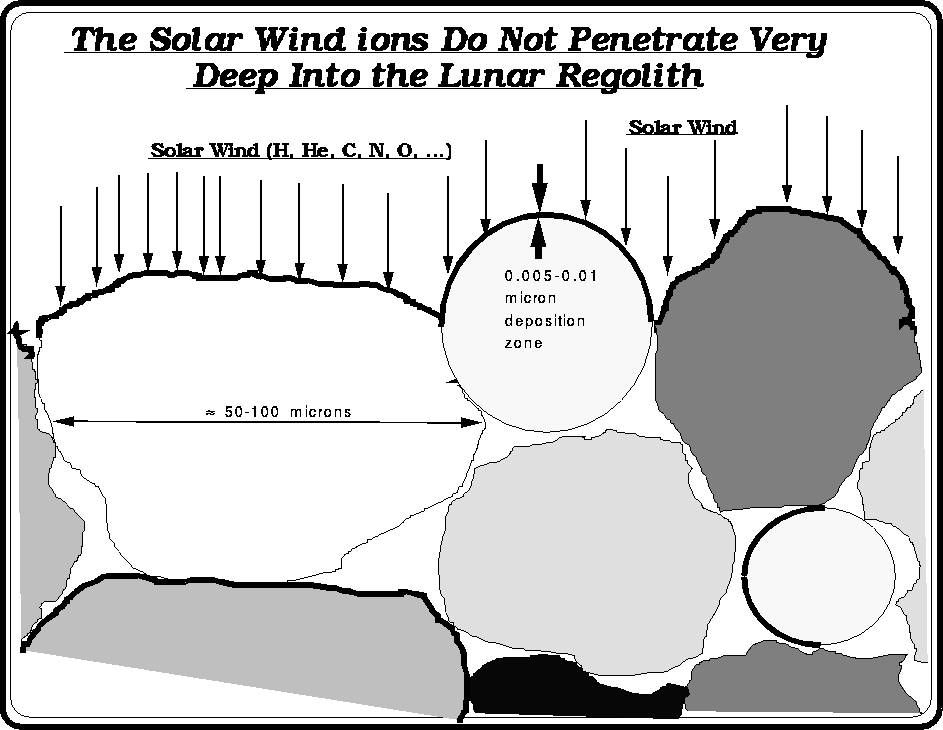
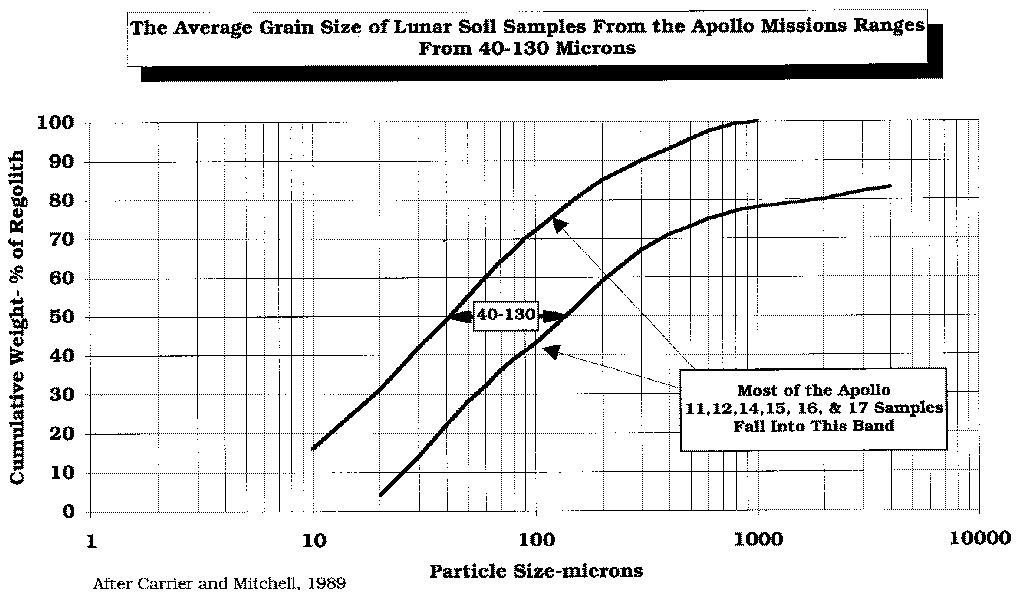 After
Carrier and Mitchell, 1989.
After
Carrier and Mitchell, 1989.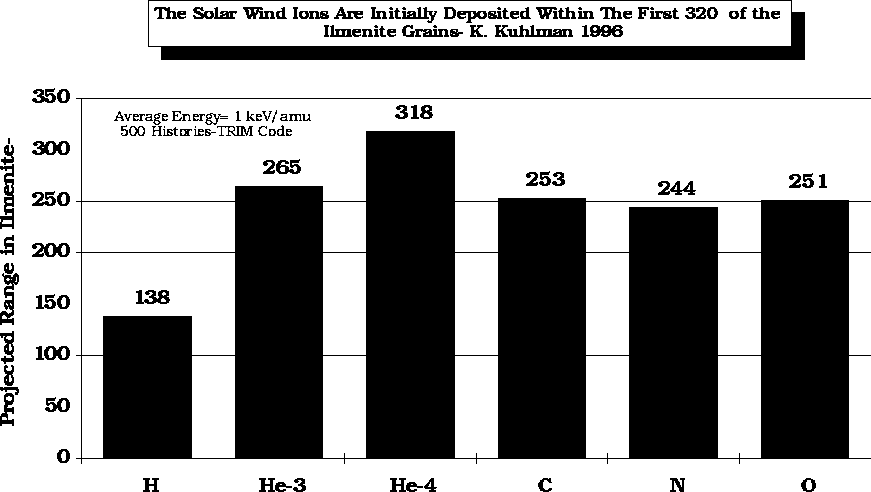
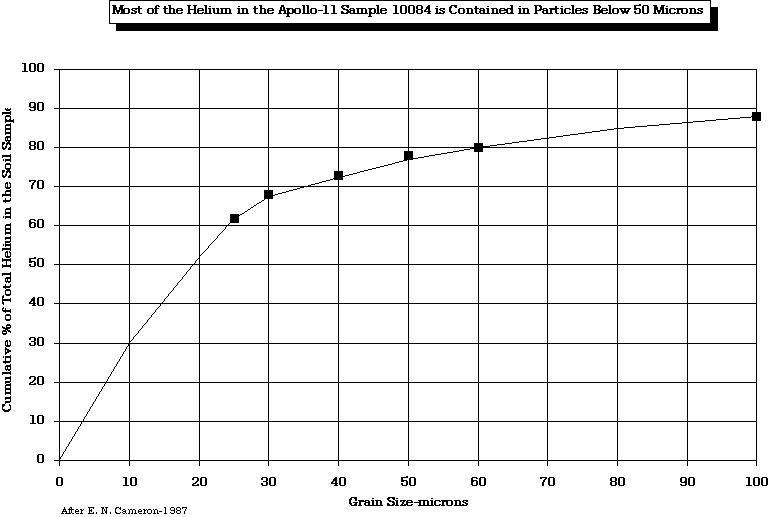
 After
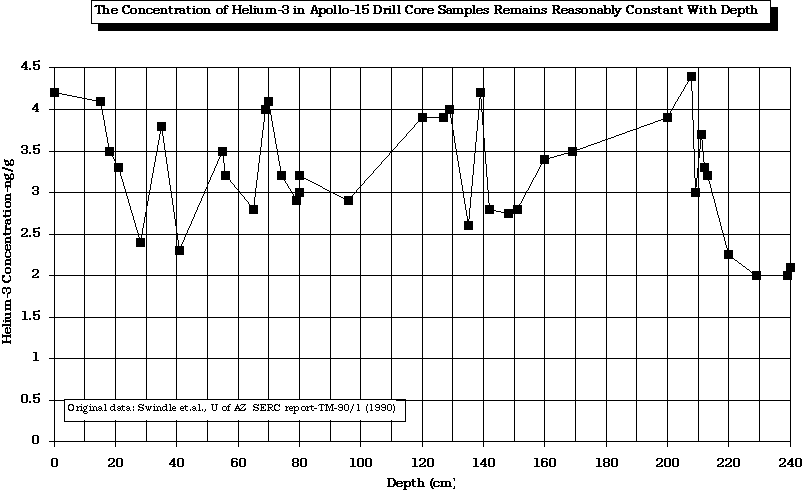
Swindle (1990)
After
Swindle (1990)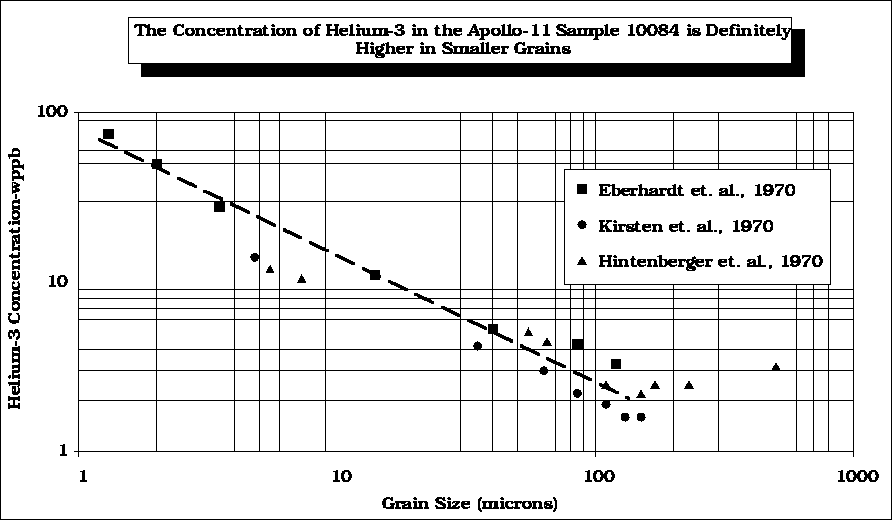
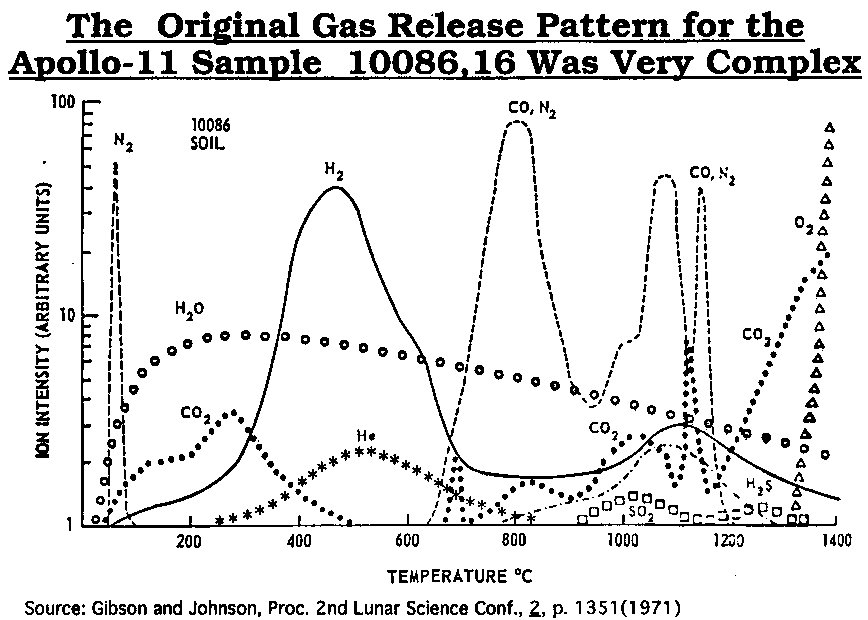 after
Gibson and Johnson (1971)
after
Gibson and Johnson (1971)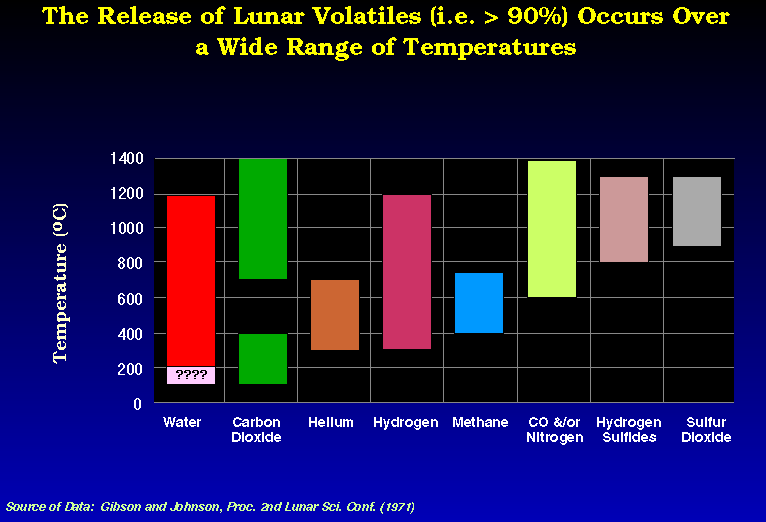 modified
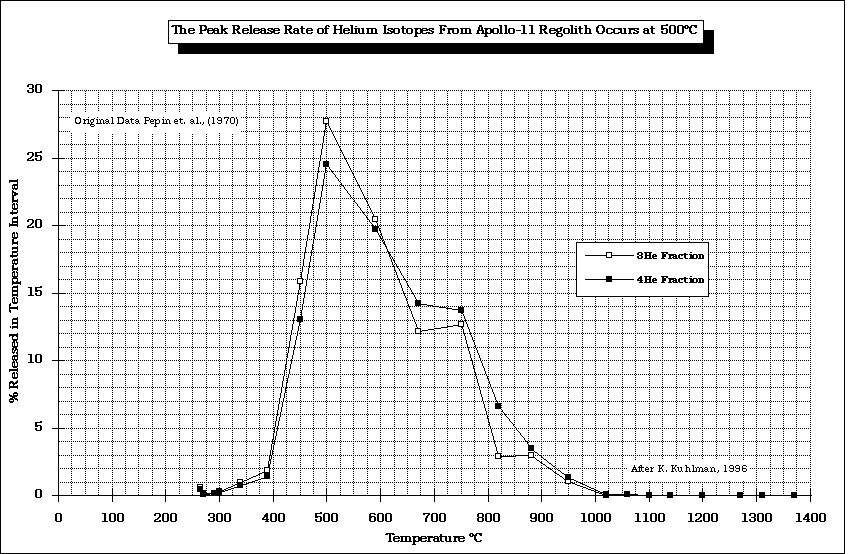
from Gibson and Johnson (1971)
modified
from Gibson and Johnson (1971)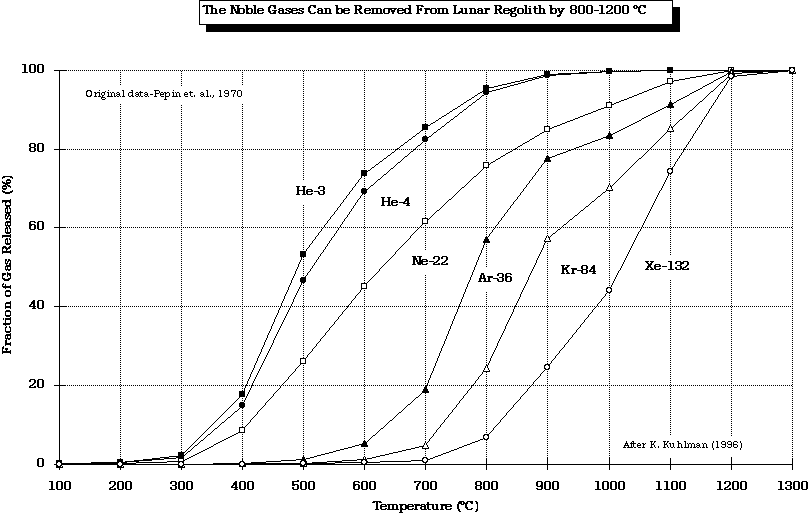
References
Bula, R. J., Wittenberg, L. J., Tibbets, T. W., and Kulcinski, G. L., 1988, "Potential of Derived Lunar Volatiles for Life Support", p. 547 in The Second Conference on Lunar Bases and Space Activities of the 21st Century, W. W. Mendell ed., NASA Conference Publication 3166, Vol. 1
Cameron, E. N., 1988, "Helium Mining on the Moon: Site Selection and Evaluation", University of Wisconsin Technical Report, WCSAR-TR-AR3-8810-6.
Cameron, E. N., 1992, "Helium Resources of Mare Tranqillitatis", University of Wisconsin Technical Report, WCSAR-TR-AR3-9207-1.
Cameron, E. N., 1993, "Evaluation of the Regolith of Mare Tranqillitatis as a Source of Volatile Elements," University of Wisconsin Technical Report, WCSAR-TR-AR3-9301-1
Carrier, W. D. III, Mitchell, J. K., 1989
Eberhardt, P., et. al., 1970, "Trapped Solar Wind Noble Gases, Exposure Age and K/Ar-age in Apollo-11 Lunar Fine Material", Proc. Apollo-11 Lunar Conf. 2:1037-1070.
Fegley, B., Jr., and Swindle, T. D., 1993, "Lunar Volatiles: Implications for Lunar Resource Utilization", in Lewis, J., Matthews, M.S., and Guerrieri, M. L., 1993, Editors, Resources of Near-Earth Space, University of Arizona Press.
Gibson, E. K. Jr., and Johnson, F. S., 1971, Proceedings 2nd Lunar Sci. Conf., Vol. 2, p. 1351.
Haskin, L., 1988, "Water and Cheese From the Lunar Desert: Abundances and Accessibility of H, C, and N on the Moon", P. 393 in The Second Conference on Lunar Bases and Space Activities of the 21st Century, W. W. Mendell ed., NASA Conference Publication 3166, Vol. 1
Heiken, G., et al, 1991, Lunar Source Book, Cambridge University Press, Cambridge, 736p.
Hintenberger, H., et. al., 1970, "Concentrations and Isotopic Abundances of the Rare Gases, Hydrogen and Nitrogen in Apollo-11 Lunar Matter", Proc. Apollo-11 Lunar Conf. 2:1607-1625.
Horz, F., Gibbons, R. V., Gault, D. E., Hartung, J. B., and Brownlee, D. E., 1975, "Some Correlation of Rock Exposure Ages and Regolith Dynamics", Proc. 6th Lunar Sci. Conf., p. 3495-3508.
Kirsten, T., Muller, O., Steinbruhnn, F., and Zahringer, J., 1970, "Study of Distribution and Variations of Rare Gases in Lunar Material by a Microprobe Technique", Proc. Apollo-11 Lunar Conf. 2:1331-1343.
Kuhlman, K., 1996, unpublished data.
Pepin, R. O., Nyquist, L. E., Phinney, D., and Black, D. C., 1970, "Rare Gases in Apollo 11 Lunar Material", Proc. Apollo 11 Lunar Sci. Conf., pp. 1443-1454
Swindle, T. D., Glass, C. E., and Poulton, M. M., 1990, "Mining Lunar Soils for 3He", UA/NASA Space Engineering Research Center TM-90/1 (Tucson: UA/NASA SERC).
Taylor, G. J., 1995, Univ. of Hawaii, artwork on the food and soft drinks
Representative Questions
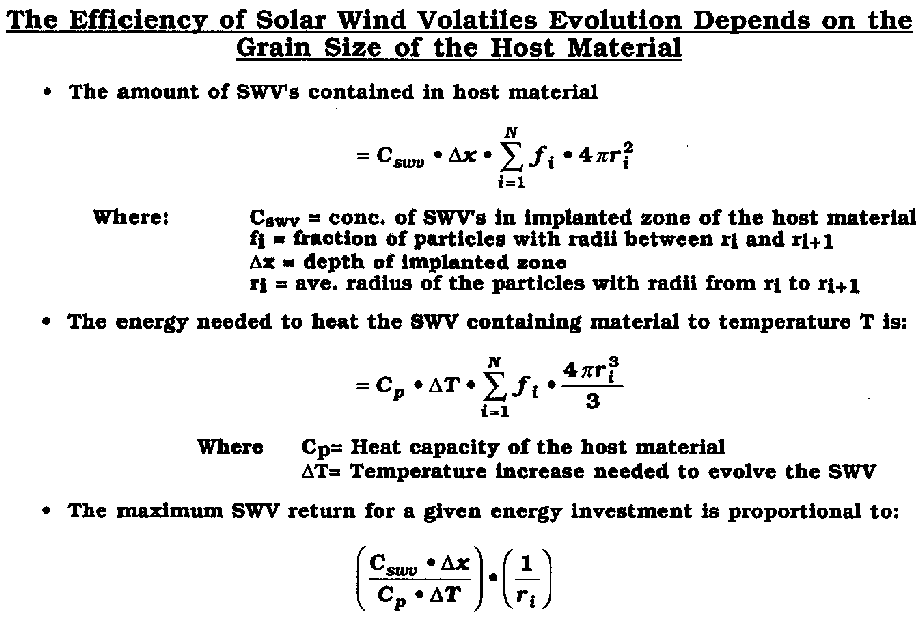
1.) Compare the energy required to extract 1 kg of 3He from lunar regolith at 700 oC to that required at 900 oC. Use the data presented in class for the evolution as a function of temperature and be clear about your assumptions on the thermal properties of lunar regolith
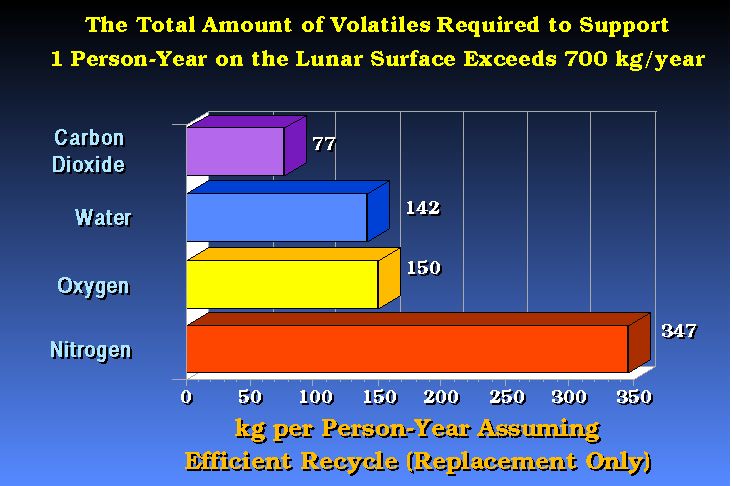
2.) Reproduce L. Haskin's calculation on the amount of food one could produce from the Solar Wind Volatiles in 1 m3 of lunar regolith.
3.) Discuss the engineering considerations that you would have to consider if you wanted to gather the maximum amount of nitrogen from lunar regolith.
 |
|
University of Wisconsin Fusion Technology Institute · 439 Engineering Research Building · 1500 Engineering Drive · Madison WI 53706-1609 · Telephone: (608) 263-2352 · Fax: (608) 263-4499 · Email: fti@engr.wisc.edu |
Copyright © 2003 The Board of
Regents of the University of Wisconsin System.
For feedback or accessibility issues, contact
web@fti.neep.wisc.edu.
|