NEEP602 Course Notes (Spring 1996)
Resources from Space
Next: Objectives of an Up: Table of Contents Previous: Introduction
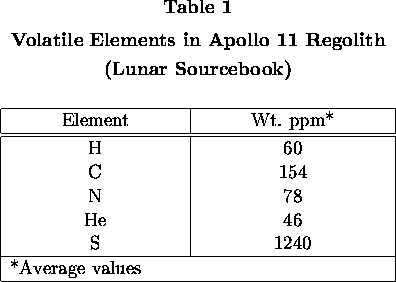
Concentrations of Various Volatiles in Apollo 11 Regolith
Concentrations of important volatile elements in Apollo 11 regolith as
indicated by analyses of various samples of regolith fines are given in Table 1.
Hydrogen, helium, carbon, and nitrogen are derived mainly or entirely from the
solar wind. Sulfur is derived at least mainly from sulfides in regolith
particles. It is now established that concentrations of solar wind gases in
regoliths are a function of maturity, which in turn is a function of length of
exposure to the solar wind. It is also established that the helium content of
regolith is a function of the ilmenite content, hence in general to the TiO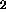 content. Bustin and Gibson (1992) conclude that the hydrogen content is also
related to the TiO
content. Bustin and Gibson (1992) conclude that the hydrogen content is also
related to the TiO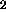 content, but this is not clear from the data presented in
their Table 1. Sample 75261, mature regolith from Apollo 17 containing
9-10% TiO
content, but this is not clear from the data presented in
their Table 1. Sample 75261, mature regolith from Apollo 17 containing
9-10% TiO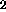 , is reported to contain 60.2 wppm H. However, sample 15261,
with only 1.50% TiO
, is reported to contain 60.2 wppm H. However, sample 15261,
with only 1.50% TiO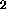 , is reported to contain 58.2 wppm H. The data of
their table suggest that H content of regolith is far more a function of
maturity
than of TiO
, is reported to contain 58.2 wppm H. The data of
their table suggest that H content of regolith is far more a function of
maturity
than of TiO content.
content.
Release of various gases from Apollo 11 fines on heating has been studied by
Gibson and Johnson (1971). Solar wind helium is almost entirely released
between 300o and 700oC, along with most of the hydrogen. The
ratio
of H to He in regolith (7-7.5) is lower than the ratio in the solar wind (17),
possibly due to diffusion of H from the samples.
from the samples.
At temperatures below 500oC, CO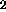 is the major C-containing gaseous
phase. Its source is uncertain. CO
is the major C-containing gaseous
phase. Its source is uncertain. CO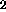 is further released at 675oC and
1125oC, and again above 1200oC. At temperatures above
700oC the principal gaseous phase released is mass 28, CO and/or N
is further released at 675oC and
1125oC, and again above 1200oC. At temperatures above
700oC the principal gaseous phase released is mass 28, CO and/or N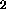 .
The CO is believed derived by reaction of C-containing phases, such as cohenite
[(Fe, Ni)3C], with silicates.
.
The CO is believed derived by reaction of C-containing phases, such as cohenite
[(Fe, Ni)3C], with silicates.
Sulfur is released as H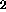 S and SO
S and SO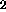 . Evolution of the two gases begins
above 900oC, which means that they will not be present during heating of
the soil for release of hydrogen and helium. Most of the sulfur is evolved
above
1,000oC.
. Evolution of the two gases begins
above 900oC, which means that they will not be present during heating of
the soil for release of hydrogen and helium. Most of the sulfur is evolved
above
1,000oC.
As indicated in my previous report, various studies have shown that the solar wind gases are heavily concentrated in the finer size fractions of the regolith, since implantation of the gases is a surface phenomenon and is therefore proportional to particle surface area per unit of mass. This must be taken into account in devising and testing methods of processing the regolith on the Moon.
Next: Objectives of an Up: Table of Contents Previous: Introduction
WCSAR-TR-AR3-9301-1
Eugene N. Cameron
Back to Lecture 19 (fall'96)
Back to Lecture 13 (fall'96)
Back to Lecture 13 (fall'97)
 |
|
University of Wisconsin Fusion Technology Institute · 439 Engineering Research Building · 1500 Engineering Drive · Madison WI 53706-1609 · Telephone: (608) 263-2352 · Fax: (608) 263-4499 · Email: fti@engr.wisc.edu |
Copyright © 2003 The Board of
Regents of the University of Wisconsin System.
For feedback or accessibility issues, contact
web@fti.neep.wisc.edu.
|